Abstract
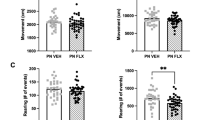
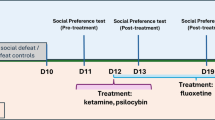
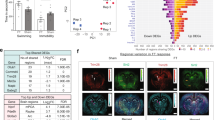
Selective serotonin reuptake inhibitors (SSRIs) are widely used to treat psychiatric disorders with affective biases such as depression and anxiety. How SSRIs exert a beneficial action on emotions associated with life events is still unknown. Here we ask whether and how the effectiveness of the SSRI fluoxetine is underpinned by neural mechanisms in the ventral striatum. To address these issues, we studied the spiking activity of neurons in the ventral striatum of monkeys during an approach-avoidance task in which the valence assigned to sensory stimuli was manipulated. Neural responses to positive and negative events were measured before and during a 4-week treatment with fluoxetine. We conducted PET scans to confirm that fluoxetine binds within the ventral striatum at a therapeutic dose. In our monkeys, fluoxetine facilitated approach of rewards and avoidance of punishments. These beneficial effects were associated with changes in tonic and phasic activities of striatal neurons. Fluoxetine increased the spontaneous firing rate of striatal neurons and amplified the number of cells responding to rewards versus punishments, reflecting a drug-induced positive shift in the processing of emotionally valenced information. These findings reveal how SSRI treatment affects ventral striatum neurons encoding positive and negative valence and striatal signaling of emotional information. In addition to a key role in appetitive processing, our results shed light on the involvement of the ventral striatum in aversive processing. Together, the ventral striatum appears to play a central role in the action of SSRIs on emotion processing biases commonly observed in psychiatric disorders.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Artigas F, Romero L, de Montigny C, Blier P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci. 1996;19:378–83.
Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O’Leary OF, et al. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–8.
Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–9.
Warren MB, Pringle A, Harmer CJ. A neurocognitive model for understanding treatment action in depression. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140213.
Harmer CJ, Duman RS, Cowen PJ. How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry. 2017;4:409–18.
Roiser JP, Elliott R, Sahakian BJ. Cognitive mechanisms of treatment in depression. Neuropsychopharmacology. 2012;37:117–36.
Harmer CJ, Bhagwagar Z, Perrett DI, Völlm BA, Cowen PJ, Goodwin GM. Acute SSRI administration affects the processing of social cues in healthy volunteers. Neuropsychopharmacology. 2003;28:148–52.
Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry. 2006;59:816–20.
Harmer CJ, O’Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A, et al. Effect of acute antidepressant administration on negative affective bias in depressed patients. Am J Psychiatry. 2009;166:1178–84.
Harmer CJ, Shelley NC, Cowen PJ, Goodwin GM. Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. Am J Psychiatry. 2004;161:1256–63.
Shiroma PR, Thuras P, Johns B, Lim KO. Emotion recognition processing as early predictor of response to 8-week citalopram treatment in late-life depression. Int J Geriatr Psychiatry. 2014;29:1132–9.
Harmer CJ, Rogers RD, Tunbridge E, Cowen PJ, Goodwin GM. Tryptophan depletion decreases the recognition of fear in female volunteers. Psychopharmacology. 2003;167:411–7.
Tranter R, Bell D, Gutting P, Harmer C, Healy D, Anderson IM. The effect of serotonergic and noradrenergic antidepressants on face emotion processing in depressed patients. J Affect Disord. 2009;118:87–93.
Bigos KL, Pollock BG, Aizenstein HJ, Fisher PM, Bies RR, Hariri AR. Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharmacology. 2008;33:3221–5.
Del-Ben CM, Deakin JFW, McKie S, Delvai NA, Williams SR, Elliott R, et al. The effect of citalopram pretreatment on neuronal responses to neuropsychological tasks in normal volunteers: an FMRI study. Neuropsychopharmacology. 2005;30:1724–34.
Murphy SE, Norbury R, O’Sullivan U, Cowen PJ, Harmer CJ. Effect of a single dose of citalopram on amygdala response to emotional faces. Br J Psychiatry J Ment Sci. 2009;194:535–40.
Outhred T, Das P, Felmingham KL, Bryant RA, Nathan PJ, Malhi GS, et al. Impact of acute administration of escitalopram on the processing of emotional and neutral images: a randomized crossover fMRI study of healthy women. J Psychiatry Neurosci. 2014;39:267–75.
Brühl AB, Jäncke L, Herwig U. Differential modulation of emotion processing brain regions by noradrenergic and serotonergic antidepressants. Psychopharmacology. 2011;216:389–99.
Brühl AB, Kaffenberger T, Herwig U. Serotonergic and noradrenergic modulation of emotion processing by single dose antidepressants. Neuropsychopharmacology. 2010;35:521–33.
Ma Y. Neuropsychological mechanism underlying antidepressant effect: a systematic meta-analysis. Mol Psychiatry. 2015;20:311–9.
Simmons AN, Arce E, Lovero KL, Stein MB, Paulus MP. Subchronic SSRI administration reduces insula response during affective anticipation in healthy volunteers. Int J Neuropsychopharmacol. 2009;12:1009–20.
Vai B, Bulgarelli C, Godlewska BR, Cowen PJ, Benedetti F, Harmer CJ. Fronto-limbic effective connectivity as possible predictor of antidepressant response to SSRI administration. Eur Neuropsychopharmacology. 2016;26:2000–10.
Choi EY, Ding S-L, Haber SN. Combinatorial Inputs to the ventral striatum from the temporal cortex, frontal cortex, and amygdala: implications for segmenting the striatum. ENeuro. 2017;4.
Pohlack ST, Nees F, Ruttorf M, Schad LR, Flor H. Activation of the ventral striatum during aversive contextual conditioning in humans. Biol Psychol. 2012;91:74–80.
Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40:1251–7.
Van Bockstaele EJ, Biswas A, Pickel VM. Topography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens. Brain Res. 1993;624:188–98.
Parent A, Mackey A, De, Bellefeuille L. The subcortical afferents to caudate nucleus and putamen in primate: a fluorescence retrograde double labeling study. Neuroscience. 1983;10:1137–50.
Beliveau V, Ganz M, Feng L, Ozenne B, Højgaard L, Fisher PM, et al. A high-resolution in vivo atlas of the human brain’s serotonin system. J Neurosci J Soc Neurosci. 2017;37:120–8.
Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26.
Schultz W. Reward functions of the basal ganglia. J Neural Transm. 2016;123:679–93.
Saga Y, Ruff CC, Tremblay L. Disturbance of approach-avoidance behaviors in non-human primates by stimulation of the limbic territories of basal ganglia and anterior insula. Eur J Neurosci. 2019;49:687–700.
Worbe Y, Baup N, Grabli D, Chaigneau M, Mounayar S, McCairn K, et al. Behavioral and movement disorders induced by local inhibitory dysfunction in primate striatum. Cereb Cortex. 2009;19:1844–56.
Worbe Y, Epinat J, Féger J, Tremblay L. Discontinuous long-train stimulation in the anterior striatum in monkeys induces abnormal behavioral states. Cereb Cortex. 2011;21:2733–41.
Tremblay L, Worbe Y, Thobois S, Sgambato-Faure V, Féger J. Selective dysfunction of basal ganglia subterritories: from movement to behavioral disorders. Mov Disord. 2015;30:1155–70.
Sgambato-Faure V, Worbe Y, Epinat J, Féger J, Tremblay L. Cortico-basal ganglia circuits involved in different motivation disorders in non-human primates. Brain Struct Funct. 2016;221:345–64.
Saga Y, Richard A, Sgambato-Faure V, Hoshi E, Tobler PN, Tremblay L. Ventral pallidum encodes contextual information and controls aversive behaviors. Cereb Cortex. 2017;27:2528–43.
Fontenot MB, Padgett EE, Dupuy AM, Lynch CR, De Petrillo PB, Higley JD. The effects of fluoxetine and buspirone on self-injurious and stereotypic behavior in adult male rhesus macaques. Comp Med. 2005;55:67–74.
Fontenot MB, Musso MW, McFatter RM, Anderson GM. Dose-finding study of fluoxetine and venlafaxine for the treatment of self-injurious and stereotypic behavior in rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci. 2009;48:176–84.
Adler A, Katabi S, Finkes I, Prut Y, Bergman H. Different correlation patterns of cholinergic and GABAergic interneurons with striatal projection neurons. Front Syst Neurosci. 2013;7:47.
Joshua M, Adler A, Mitelman R, Vaadia E, Bergman H. Midbrain dopaminergic neurons and striatal cholinergic interneurons encode the difference between reward and aversive events at different epochs of probabilistic classical conditioning trials. J Neurosci. 2008;28:11673–84.
Berke JD, Okatan M, Skurski J, Eichenbaum HB. Oscillatory entrainment of striatal neurons in freely moving rats. Neuron. 2004;43:883–96.
Golub MS, Hogrefe CE, Sherwood RJ, Turck CW. Fluoxetine administration in juvenile monkeys: implications for pharmacotherapy in children. Front Pediatr. 2018;6:21.
Beaudoin-Gobert M, Epinat J, Météreau E, Duperrier S, Neumane S, Ballanger B, et al. Behavioural impact of a double dopaminergic and serotonergic lesion in the non-human primate. Brain J Neurol. 2015;138:2632–47.
Millot M, Saga Y, Duperrier S, Météreau E, Beaudoin-Gobert M, Sgambato V. Prior MDMA administration aggravates MPTP-induced Parkinsonism in macaque monkeys. Neurobiol Dis. 2020;134:104643.
Ballanger B, Tremblay L, Sgambato-Faure V, Beaudoin-Gobert M, Lavenne F, Le Bars D, et al. A multi-atlas based method for automated anatomical Macaca fascicularis brain MRI segmentation and PET kinetic extraction. NeuroImage. 2013;77:26–43.
Pasquereau B, Turner RS. Primary motor cortex of the parkinsonian monkey: differential effects on the spontaneous activity of pyramidal tract-type neurons. Cereb Cortex. 2011;21:1362–78.
Bradley BP, Mogg K, Williams R. Implicit and explicit memory for emotion-congruent information in clinical depression and anxiety. Behav Res Ther. 1995;33:755–70.
Heuer K, Rinck M, Becker ES. Avoidance of emotional facial expressions in social anxiety: The Approach-Avoidance Task. Behav Res Ther. 2007;45:2990–3001.
Vrijsen JN, van Oostrom I, Speckens A, Becker ES, Rinck M. Approach and avoidance of emotional faces in happy and sad mood. Cogn Ther Res. 2013;37:1–6.
Bourke C, Douglas K, Porter R. Processing of facial emotion expression in major depression: a review. Aust N Z J Psychiatry. 2010;44:681–96.
Carlisi CO, Robinson OJ. The role of prefrontal-subcortical circuitry in negative bias in anxiety: Translational, developmental and treatment perspectives. Brain Neurosci Adv. 2018;2:2398212818774223.
Bouhuys AL, Geerts E, Gordijn MC. Depressed patients’ perceptions of facial emotions in depressed and remitted states are associated with relapse: a longitudinal study. J Nerv Ment Dis. 1999;187:595–602.
Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive therapy of depression. 13. Guilford Press: New York; 1979.
Fava M. Symptoms of fatigue and cognitive/executive dysfunction in major depressive disorder before and after antidepressant treatment. J Clin Psychiatry. 2003;64:30–34.
Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40.
Anderson IM, Del-Ben CM, Mckie S, Richardson P, Williams SR, Elliott R, et al. Citalopram modulation of neuronal responses to aversive face emotions: a functional MRI study. Neuroreport. 2007;18:1351–5.
Fu CHY, Williams SCR, Cleare AJ, Brammer MJ, Walsh ND, Kim J, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:877–89.
Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–8.
Chen C-H, Suckling J, Ooi C, Fu CHY, Williams SCR, Walsh ND, et al. Functional coupling of the amygdala in depressed patients treated with antidepressant medication. Neuropsychopharmacology. 2008;33:1909–18.
Stoy M, Schlagenhauf F, Sterzer P, Bermpohl F, Hägele C, Suchotzki K, et al. Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. J Psychopharmacol. 2012;26:677–88.
Ossewaarde L, Verkes RJ, Hermans EJ, Kooijman SC, Urner M, Tendolkar I, et al. Two-week administration of the combined serotonin-noradrenaline reuptake inhibitor duloxetine augments functioning of mesolimbic incentive processing circuits. Biol Psychiatry. 2011;70:568–74.
McCabe C, Mishor Z, Cowen PJ, Harmer CJ. Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol Psychiatry. 2010;67:439–45.
Heller AS, Johnstone T, Light SN, Peterson MJ, Kolden GG, Kalin NH, et al. Relationships between changes in sustained fronto-striatal connectivity and positive affect in major depression resulting from antidepressant treatment. Am J Psychiatry. 2013;170:197–206.
Takamura M, Okamoto Y, Okada G, Toki S, Yamamoto T, Ichikawa N, et al. Patients with major depressive disorder exhibit reduced reward size coding in the striatum. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79:317–23.
Bissonette GB, Burton AC, Gentry RN, Goldstein BL, Hearn TN, Barnett BR, et al. Separate populations of neurons in ventral striatum encode value and motivation. PLoS ONE. 2013;8:e64673.
Reynolds SM, Berridge KC. Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nat Neurosci. 2008;11:423–5.
Ironside M, Amemori K-I, McGrath CL, Pedersen ML, Kang MS, Amemori S, et al. Approach-avoidance conflict in major depressive disorder: congruent neural findings in humans and nonhuman primates. Biol Psychiatry. 2020;87:399–408.
Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci. 2012;13:251–66.
Sgambato-Faure V, Tremblay L. Dopamine and serotonin modulation of motor and non-motor functions of the non-human primate striato-pallidal circuits in normal and pathological states. J Neural Transm 2018;125:485–500.
Amemori K-I, Amemori S, Gibson DJ, Graybiel AM. Striatal microstimulation induces persistent and repetitive negative decision-making predicted by striatal beta-band oscillation. Neuron. 2018;99:829–41.e6.
Acknowledgements
We are grateful to Robert S. Turner and Sylvia Wirth for helpful comments on the manuscript.
Author information
Authors and Affiliations
Contributions
LT, PNT and VS designed the study. GD, YS and AR collected the electrophysiological data. MM and EM collected and processed imaging data. BP was primary analyzer of the data and writer of the manuscript.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Pasquereau, B., Drui, G., Saga, Y. et al. Selective serotonin reuptake inhibitor treatment retunes emotional valence in primate ventral striatum. Neuropsychopharmacol. 46, 2073–2082 (2021). https://doi.org/10.1038/s41386-021-00991-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-021-00991-x
This article is cited by
-
Positive bias in brain and behaviour as a mechanism of transcranial magnetic stimulation depression treatment
Molecular Psychiatry (2026)
-
Acute effects of selective serotonin reuptake inhibitors on cerebral glucose metabolism and blood flow
Translational Psychiatry (2026)
-
Suprachiasmatic nucleus dysfunction induces anxiety- and depression-like behaviors via activating the BDNF-TrkB pathway of the striatum
Translational Psychiatry (2025)
-
Dopamine and serotonin neurotransmissions exert complementary control over primate approach and avoidance
Translational Psychiatry (2025)
-
Fluoxetine incentivizes ventral striatum encoding of reward and punishment
Neuropsychopharmacology (2021)