Abstract
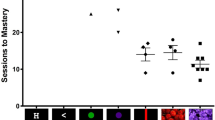
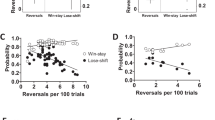
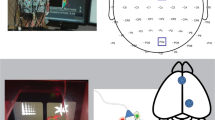
Progress towards understanding neural mechanisms in humans relevant to psychiatric conditions has been hindered by a lack of translationally-relevant cognitive tasks for laboratory animals. Accordingly, there is a critical need to develop parallel neurophysiological assessments of domains of cognition, such as cognitive control, in humans and laboratory animals. To address this, we developed a touchscreen-based cognitive (Eriksen Flanker) task in rats and used its key characteristics to construct a novel human version, with similar testing parameters and endpoints across species. We obtained continuous electroencephalogram (EEG) recordings, including local field potentials in rats, and compared electrophysiological signatures locked to stimulus onset and responses across species. We also assessed whether behavioral or physiological task effects were modulated by modafinil, which enhances aspects of cognitive function in humans. In both species, the task elicited expected flanker interference effects (reduced accuracy) during high-conflict trials. Across homologous neuroanatomical loci, stimulus-locked increases in theta power during high-conflict trials as well as error-related negative potentials were observed. These endpoints were not affected by modafinil in either species. Despite some species-specific patterns, our findings demonstrate the feasibility of a rat Flanker task as well as cross-species behavioral and neurophysiological similarities, which may enable novel insights into the neural correlates of healthy and aberrant behavior and provide mechanistic insights relevant to treatment.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Substance Abuse and Mental Health Services Administration. (2018) Key substance use and mental health indicators in the United States: results from the 2017 National Survey on Drug Use and Health (HHS Publication No. SMA 18-5068, NSUDH Series H-53). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration.
Deacon BJ. The biomedical model of mental disorder: a critical analysis of its validity, utility and effects on psychotherapy research. Clin Psych Rev. 2013;33:846–61.
Bale TL, Abel T, Akil H, Carlezon WA Jr, Moghaddam B, Nestler EJ, et al. The critical importance of basic animal research for neuropsychiatric disorders. Neuropsychopharmacology. 2019;44:1349–53.
Hyman SE. Revitalizing psychiatric therapeutics. Neuropsychopharmacology. 2014;39:220–9.
Insel TR, Cuthbert BN, Garvey MA, Heinssen RK, Pine DS. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–51.
Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16:143–9.
Gehring WJ, Liu Y, Orr JM, & Carp J. The error-related negativity (ERN/Ne). In: Luck SJ, Kappenman ES, editors. The oxford handbook of event-related potential components. Oxford: Oxford University Press; 2012. p 231–91.
Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci. 2014;18:414–21.
Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–52.
Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–22.
Holroyd CB, Yeung N. Motivation of extended behaviors by anterior cingulate cortex. Trends Cogn Sci. 2012;16:122–8.
Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain, and cognitive control in cingulate cortex. Nat Rev Neurosci. 2011;12:154–67.
Holmes AJ, Pizzagalli DA. Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Arch Gen Psychiatry. 2008;65:179–88.
Bates AT, Kiehl KA, Laurens KR, Liddle PF. Error-related negativity and correct response negativity in schizophrenia. Clin Neurophysiol. 2002;113:1454–63.
Paulus MP, Feinstein JS, Simmons A, Stein MB. Anterior cingulate activation in high trait anxious subjects is related to altered error processing during decision making. Biol Psychol. 2004;55:1179–87.
Narayanan NS, Cavanagh JF, Frank MJ, Laubach M. Common medial frontal mechanisms of adaptive control in humans and rodents. Nat Neurosci. 2013;16:1888–95.
Abram SV, Breton YA, Schmidt B, Redish AD, MacDonald AW. The web-surf task: a translational model of human decision-making. Cogn Aff Behav Neurosci. 2016;16:37–50.
Hyman JM, Holroyd CB, Seamans JK. A novel neural prediction error found in anterior cingulate cortex ensembles. Neuron. 2017;95:447–56.
Bussey TJ, Holmes A, Lyon L, Mar AC, McAllister KAL, Nithianantharajah J, et al. New translational assays for preclinical modelling of cognition in schizophrenia: the touchscreen testing method for mice and rats. Neuropharmacology. 2012;62:1191–203.
Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–81.
Gehring WJ, Goss B, Coles MG, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–90.
Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Steola V, et al. Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J Pharm Exp Ther. 2009;329:738–46.
Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects oncognition. Neuropsychopharmacology. 2008;33:1477–502.
Olvet DM, Hajcak G. The stability of error-related brain activity with increasing trials. Psychophysiology. 2009;46:957–61.
First MB, Williams JBW, Karg RS, & Spitzer RL. Structured clinical interview for DSM-5 Research version (SCID-5 for DSM-5, research version; SCID-5-RV). Arlington, VA: American Psychiatric Association: 2015. p. 1–94.
Robertson P, Hellriegel ET. Clinical pharmacokinetic profile of modafinil. Clin Pharmacokinet. 2003;42:123–37.
Schroder HS, Nickels S, Cardenas E, Breiger M, Perlo S, Pizzagalli DA. Optimizing assessments of post-error slowing: a neurobehavioral investigation of a flanker task. Psychophysiology. 2020;57:e13473.
Peirce JW. PsychoPy—psychophysics software in Python. J Neurosci Methods. 2007;162:8–13.
Carlezon WA, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, et al. Depressive-like effects of the κ-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharm Exp Ther. 2006;316:440–7.
Nemeth CL, Paine TA, Rittiner JE, Béguin C, Carroll FI, Roth BL, et al. Role of kappa-opioid receptors in the effects of salvinorin A and ketamine on attention in rats. Psychopharmacology. 2010;210:263–74.
Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 6th ed. Elsevier; 2006.
Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev. 2004;111:931.
Cohen, MX. Analyzing neural time series data: theory and practice. Cambridge, MA: MIT Press; 2014.
Tsano M, Chah E, Vann SD, Reilly RB, Erichsen JT, Aggleton JP, et al. Theta-modulated head direction cells in the rat anterior thalamus. J Neurosci. 2011;31:9489–502.
Martínez‐Bellver S, Cervera‐Ferri A, Luque‐García A, Martínez‐Ricós J, Valverde Navarro A, Bataller M, et al. Causal relationships between neurons of the nucleus incertus and the hippocampal theta activity in the rat. J Physiol. 2017;595:1775–92. (2017)
Van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav. 2002;77:477–82.
Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol. 2002;24:5–12.
Luck SJ. An introduction to the event-related potential technique, 2nd ed. Boston, MA: MIT Press; 2014.
Overbeek TJ, Nieuwenhuis S, Ridderinkhof KR. Dissociable components of error processing: on the functional significance of the Pe vis-à-vis the ERN/Ne. J Psychophysiol. 2005;19:319–29.
Holroyd CB, Coles MGH. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109:679–709.
Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202.
Minzenberg MJ, Gomes GC, Yoon JH, Watrous AJ, Geng J, Firl AJ, et al. Modafinil augments oscillatory power in middle frequencies during rule selection. Psychophysiology. 2014;51:510–9. (2014)
Minzenberg MJ, Yoon JH, Cheng Y, Carter CS. Modafinil effects on middle frequency oscillatory power during rule selection in schizophrenia. Neuropsychopharmacology. 2014;39:3018.
Marx C, Lex B, Calaminus C, Hauber W, Backes H, Neumaier B, et al. Conflict processing in the rat brain: behavioral analysis and functional μPET imaging using [18F] fluorodeoxyglucose. Front Behav Neurosci. 2012;6:4.
Cohen MX. A neural microcircuit for cognitive conflict detection and signaling. Trends Neurosci. 2014;37:480–90. (2014)
Warren CM, Hyman JM, Seamans JK, Holroyd CB. Feedback-related negativity observed in rodent anterior cingulate cortex. J Physiol-Paris. 2015;109:87–94.
Holroyd CB, Krigolson OE, Baker R, Lee S, Gibson J. When is an error not a prediction error? An electrophysiological investigation. Cogn Aff Behav Neurosci. 2009;9:59–70.
Sambeth A, Maes JHR, Luijtelaar GV, Molenkamp IB, Jongsma ML, Rijn CMV. Auditory event–related potentials in humans and rats: Eeffects of task manipulation. Psychophysiology. 2003;40:60–68.
Gandal MJ, Edgar JC, Ehrlichman RS, Mehta M, Roberts TP, Siegel SJ. Validating γ oscillations and delayed auditory responses as translational biomarkers of autism. Biol Psychol. 2010;68:1100–6.
Fujisawa S, Buzsáki G. A 4 Hz oscillation adaptively synchronizes prefrontal, VTA, and hippocampal activities. Neuron. 2011;72:153–65.
Fu Y, Chen YM, Zeng T, Peng YP, Tian SH, Ma YY, et al. Activity in left orbitofrontal cortex in rats related to food reward and craving. J Zoo Res. 2008;3:260–4.
Acknowledgements
We would like to acknowledge the members of our scientific advisory board, Dr. Cindy Ehlers, Dr. Stan Floresco, Dr. Patricio O’Donnell, and Dr. Steven Siegel, for their assistance in the development and execution of these studies. We would also like to thank Dr. Jennifer Evans for her comments on the manuscript and Dr. David P. Olson for assistance with the medical components of the study. Finally, we would like to acknowledge Dr. Boyu Ren for his assistance with statistical analyses.
Author information
Authors and Affiliations
Contributions
MAR—design of the work. Acquisition, analysis, and interpretation of data. Drafting of manuscript. HSS—design of the work. Acquisition, analysis, and interpretation of data. Drafting of manuscript. BDK—design of the work. Interpretation of data. Revision of the manuscript. SN—design of the work. Acquisition, analysis, and interpretation of data. MB—acquisition and analysis of data. AMI-M—analysis and interpretation of data. SP—acquisition of data. EC—acquisition of data. AD-A—design of work. Interpretation of data. Revision of the manuscript. SAB—interpretation of data. Revision of the manuscript. SL—design of work. Interpretation of data. VBR—design of work. Interpretation of data. Revision of the manuscript. GV—acquisition of data, medical evaluations. Revision of the manuscript. JB—design of work. Interpretation of data. Revision of the manuscript. WAC Jr.—design of work. Interpretation of data. Revision of the manuscript. DAP—design of work. Analysis and interpretation of data. Revision of the manuscript. Secured funding.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Robble, M.A., Schroder, H.S., Kangas, B.D. et al. Concordant neurophysiological signatures of cognitive control in humans and rats. Neuropsychopharmacol. 46, 1252–1262 (2021). https://doi.org/10.1038/s41386-021-00998-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-021-00998-4
This article is cited by
-
Lost in translation: toward clinically effective translational research
Translational Psychiatry (2025)
-
Identification of conserved frontal neurophysiological markers of cognitive flexibility in humans and rats
Communications Biology (2025)
-
Comparable roles for serotonin in rats and humans for computations underlying flexible decision-making
Neuropsychopharmacology (2024)
-
Differential effects of the stress peptides PACAP and CRF on sleep architecture in mice
NPP—Digital Psychiatry and Neuroscience (2024)
-
Neurophysiological mechanisms of error monitoring in human and non-human primates
Nature Reviews Neuroscience (2023)