Abstract
Affective disorders (AD, including bipolar disorder, BD, and major depressive disorder) are severe recurrent illnesses. Identifying neural markers of processes underlying AD development in at-risk youth can provide objective, “early-warning” signs that may predate onset or worsening of symptoms. Using data (n = 34) from the Bipolar Offspring Study, we examined relationships between neural response in regions supporting executive function, and those supporting self-monitoring, during an emotional n-back task (focusing on the 2-back face distractor versus the 0-back no-face control conditions) and future depressive and hypo/manic symptoms across two groups of youth at familial risk for AD: Offspring of parents with BD (n = 15, age = 14.15) and offspring of parents with non-BD psychopathology (n = 19, age = 13.62). Participants were scanned and assessed twice, approximately 4 years apart. Across groups, less deactivation in the mid-cingulate cortex during emotional regulation (Rate Ratio = 3.07(95% CI:1.09–8.66), χ2(1) = 4.48, p = 0.03) at Time-1, and increases in functional connectivity from Time-1 to 2 (Rate Ratio = 1.45(95% CI:1.15–1.84), χ2(1) = 8.69, p = 0.003) between regions that showed deactivation during emotional regulation and the right caudate, predicted higher depression severity at Time-2. Both effects were robust to sensitivity analyses controlling for clinical characteristics. Decreases in deactivation between Times 1 and 2 in the right putamen tail were associated with increases in hypo/mania at Time-2, but this effect was not robust to sensitivity analyses. Our findings reflect neural mechanisms of risk for worsening affective symptoms, particularly depression, in youth across a range of familial risk for affective disorders. They may serve as potential objective, early-warning signs of AD in youth.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Notes
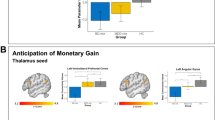
Two individuals had higher KDRS scores than the rest of the cohort. When removed from the larger models, the associations remained significant: BOLD response in the mid-cingulate continued to predict Time-2 KDRS (RR = 9.15, χ2 (1) = 6.34, p = 0.01), as did change in connectivity between the task-negative seeds and the right caudate between Time-1 and Time-2 (RR = 1.51, χ2 (1) = 6.47, p = 0.01).
References
Bostwick JM, Pankratz VS. Affective disorders and suicide risk: a reexamination. A J Psychiatry. 2000;157:1925–32.
Liu Q, He H, Yang J, Feng X, Zhao F, Lyu J. Changes in the global burden of depression from 1990 to 2017: findings from the Global Burden of Disease study. J Psychiatr Res. 2020;126:134–40.
Ferrari AJ, Stockings E, Khoo J-P, Erskine HE, Degenhardt L, Vos T, et al. The prevalence and burden of bipolar disorder: Findings from the Global Burden of Disease Study 2013. Bipolar Disord. 2016;18:440–50.
Burke KC, Burke JD, Regier DA, Rae DS. Age at onset of selected mental disorders in five community populations. Arch Gen Psychiatry. 1990;47:511–18.
Leibenluft E, Rich B. Pediatric bipolar disorder. Ann Rev Clin Psychol. 2008:4;163–87.
Birmaher B, Axelson D. Course and outcome of bipolar spectrum disorder in children and adolescents: a review of the existing literature. Dev Psychopathol. 2006;18:1023–35.
DelBello MP, Hanseman D, Adler CM, Fleck DE, Strakowski SM. Twelve-month outcome of adolescents with bipolar disorder following first hospitalization for a manic or mixed episode. A J Psychiatry. 2007;164:582–90.
Chang K, Howe M, Gallelli K, Miklowitz D. Prevention of pediatric bipolar disorder: Integration of neurobiological and psychosocial processes. Ann NY Acad Sci. 2006;1094:235–47.
Birmaher B Bipolar Disorders In: Martin A, Volkmar F, editors. Lewis’s Child and Adolescent Psychiatry: A Comprehensive Textbook. Philadelphia: Wolters Kluwer; 2018.
Birmaher B, Axelson D, Monk K, Kalas C, Goldstein B, Hickey MB, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: The Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry. 2009;66:287–96.
Pavuluri MN, Birmaher B, Naylor MW. Pediatric bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 2005;44:846–71.
Goodwin F, Jamison K, SNG Manic-depressive illness: Bipolar disorders and recurrent depression. Oxford University Press: New York, N.Y; 2007.
Merikangas KR, Jin R, He J-P, Kessler RC, Lee S, Sampson NA, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68:241–51.
Axelson D, Goldstein B, Goldstein T, Monk K, Yu H, Hickey MB, et al. Diagnostic precursors to bipolar disorder in offspring of parents with bipolar disorder: a longitudinal study. A J Psychiatry. 2015;172:638–46.
Birmaher B. Longitudinal course of pediatric bipolar disorder. Am J Psychiatry. 2007;16:537–39.
Chang KD, Steiner H, Ketter TA. Psychiatric phenomenology of child and adolescent bipolar offspring. J Am Acad Child Adolesc Psychiatry. 2000;39:453–60.
Chang K, Steiner H, Ketter T. Studies of offspring of parents with bipolar disorder. Am J Med Genet Part C: Semin Med Genet. 2003;123:26–35.
Parker G. Parental representations and affective symptoms: examination for an hereditary link. Br J Med Psychol. 1982;55:57–61.
Warner V, Weissman M, Fendrich M, Wickramaratne P, Moreau D. The course of major depression in the offspring of depressed parents: Incidence, recurrence, and recovery. Arch Gen Psychiatry. 1992;49:795–801.
Weissman MM, Wickramaratne P, Gameroff MJ, Warner V, Pilowsky D, Kohad RG, et al. Offspring of depressed parents: 30 years later. A J Psychiatry. 2016;173:1024–32.
Weissman MM, Wickramaratne P, Nomura Y, Warner V, Pilowsky D, Verdeli H. Offspring of depressed parents: 20 years later. A J Psychiatry. 2006;163:1001–08.
Smoller JW, Finn CT. Family, twin, and adoption studies of bipolar disorder. Am J Med Genet. 2003;123C:48–58.
Hillegers M, Reichart C, Wals M, Verhulst F, Ormel J, Nolen W. Five‐year prospective outcome of psychopathology in the adolescent offspring of bipolar parents. Bipolar Disord. 2005;7:344–50.
Duffy A. The early natural history of bipolar disorder: what we have learned from longitudinal high-risk research. Can J Psychiatry. 2010;55:477–85.
Joormann J, Gotlib IH. Emotion regulation in depression: relation to cognitive inhibition. Cognition Emot 2010;24:281–98.
Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:833–57.
Acuff H, Versace A, Bertocci M, Ladouceur C, Hanford L, Manelis A, et al. Association of neuroimaging measures of emotion processing and regulation neural circuitries with symptoms of bipolar disorder in offspring at risk for bipolar disorder. JAMA Psychiatry. 2018;75:1241–51.
Cattarinussi G, Di Giorgio A, Wolf RC, Balestrieri M, Sambataro F. Neural signatures of the risk for bipolar disorder: a meta‐analysis of structural and functional neuroimaging studies. Bipolar Disord. 2019;21:215–27.
Olsavsky AK, Brotman MA, Rutenberg JG, Muhrer EJ, Deveney CM, Fromm SJ, et al. Amygdala hyperactivation during face emotion processing in unaffected youth at risk for bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2012;51:294–303.
Tseng W-L, Bones B, Kayser R, Olsavsky A, Fromm S, Pine D, et al. An fMRI study of emotional face encoding in youth at risk for bipolar disorder. Eur Psychiatry. 2015;30:94–98.
Ladouceur CD, Farchione T, Diwadkar V, Pruitt P, Radwan J, Axelson DA, et al. Differential patterns of abnormal activity and connectivity in the amygdala-prefrontal circuitry in bipolar-I and bipolar-NOS youth. J Am Acad Child Adolesc Psychiatry. 2011;50:1275–89.e2.
Ladouceur C, Diwadkar V, White R, Bass J, Birmaher B, Axelson D, et al. Fronto-limbic function in unaffected offspring at familial risk for bipolar disorder during an emotional working memory paradigm. Developmental Cogn Neurosci. 2013;5:185–96.
Manelis A, Ladouceur CD, Graur S, Monk K, Bonar LK, Hickey MB, et al. Altered amygdala-prefrontal response to facial emotion in offspring of parents with bipolar disorder. Brain. 2015;138:2777–90.
Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–87.
Blumberg HP, Donegan NH, Sanislow CA, Collins S, Lacadie C, Skudlarski P, et al. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology. 2005;183:308–13.
Korgaonkar MS, Erlinger M, Breukelaar IA, Boyce P, Hazell P, Antees C, et al. Amygdala activation and connectivity to emotional processing distinguishes asymptomatic patients with bipolar disorders and unipolar depression. Biol Psychiatry: Cogn Neurosci Neuroimaging. 2019;4:361–70.
Drapier D, Surguladze S, Marshall N, Schulze K, Fern A, Hall M-H, et al. Genetic liability for bipolar disorder is characterized by excess frontal activation in response to a working memory task. Biol Psychiatry. 2008;64:513–20.
Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–28.
Hafeman DM, Bebko G, Bertocci MA, Fournier JC, Bonar L, Perlman SB, et al. Abnormal deactivation of the inferior frontal gyrus during implicit emotion processing in youth with bipolar disorder: attenuated by medication. J Psychiatr Res. 2014;58:129–36. (C)
Lee M-S, Anumagalla P, Talluri P, Pavuluri MN. Attentional engagement increases inferior frontal gyrus activity and mutes limbic activity in pediatric bipolar disorder: Meta-analyses of fMRI studies. Prog Neuro-Psychopharmacol Biol Psychiatry. 2019;91:14–19.
Alonso‐Lana S, Moro N, McKenna PJ, Sarró S, Romaguera A, Monté GC, et al. Longitudinal brain functional changes between mania and euthymia in bipolar disorder. Bipolar Disord. 2019;21:449–57.
Smucny J, Lesh TA, Newton K, Niendam TA, Ragland JD, Carter CS. Levels of cognitive control: a functional magnetic resonance imaging-based test of an RDoC domain across bipolar disorder and schizophrenia. Neuropsychopharmacology. 2018;43:598–606.
Borgelt L, Strakowski SM, DelBello MP, Weber W, Eliassen JC, Komoroski RA, et al. Neurophysiological effects of multiple mood episodes in bipolar disorder. Bipolar Disord. 2019;21:503–13.
Foland-Ross LC, Bookheimer SY, Lieberman MD, Sugar CA, Townsend JD, Fischer J, et al. Normal amygdala activation but deficient ventrolateral prefrontal activation in adults with bipolar disorder during euthymia. Neuroimage. 2012;59:738–44.
Townsend JD, Torrisi SJ, Lieberman MD, Sugar CA, Bookheimer SY, Altshuler LL. Frontal-amygdala connectivity alterations during emotion downregulation in bipolar I disorder. Biol Psychiatry. 2013;73:127–35.
Altshuler L, Bookheimer S, Townsend J, Proenza MA, Sabb F, Mintz J, et al. Regional brain changes in bipolar I depression: a functional magnetic resonance imaging study. Bipolar Disord. 2008;10:708–17.
Strakowski SM, Eliassen JC, Lamy M, Cerullo MA, Allendorfer JB, Madore M, et al. Functional magnetic resonance imaging brain activation in bipolar mania: evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biol Psychiatry. 2011;69:381–88.
Townsend JD, Bookheimer SY, Foland‐Ross LC, Moody TD, Eisenberger NI, Fischer JS, et al. Deficits in inferior frontal cortex activation in euthymic bipolar disorder patients during a response inhibition task. Bipolar Disord. 2012;14:442–50.
Delvecchio G, Fossati P, Boyer P, Brambilla P, Falkai P, Gruber O, et al. Common and distinct neural correlates of emotional processing in bipolar disorder and major depressive disorder: a voxel-based meta-analysis of functional magnetic resonance imaging studies. Eur Neuropsychopharmacol. 2012;22:100–13.
Passarotti AM, Ellis J, Wegbreit E, Stevens MC, Pavuluri MN. Reduced functional connectivity of prefrontal regions and amygdala within affect and working memory networks in pediatric bipolar disorder. Brain Connectivity. 2012;2:320–34.
Kim P, Thomas LA, Rosen BH, Moscicki AM, Brotman MA, Zarate J, et al. Differing amygdala responses to facial expressions in children and adults with bipolar disorder. A J Psychiatry. 2012;169:642–49.
Garrett AS, Reiss AL, Howe ME, Kelley RG, Singh MK, Adleman NE, et al. Abnormal amygdala and prefrontal cortex activation to facial expressions in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2012;51:821–31.
Ladouceur CD, Farchione T, Diwadkar V, Pruitt P, Radwan J, Axelson DA, et al. Differential patterns of abnormal activity and connectivity in the amygdala–prefrontal circuitry in bipolar-I and bipolar-NOS youth. J Am Acad Child Adolesc Psychiatry. 2011;50:1275–89. e2.
Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–47.
Taylor SF, Stern ER, Gehring WJ. Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist. 2007;13:160–72.
Piccoli T, Valente G, Linden DE, Re M, Esposito F, Sack AT, et al. The default mode network and the working memory network are not anti-correlated during all phases of a working memory task. PloS One. 2015;10:e0123354.
Tomasi D, Ernst T, Caparelli EC, Chang L. Common deactivation patterns during working memory and visual attention tasks: an intra‐subject fMRI study at 4 Tesla. Hum Brain Mapp. 2006;27:694–705.
Zuo N, Salami A, Yang Y, Yang Z, Sui J, Jiang T. Activation‐based association profiles differentiate network roles across cognitive loads. Hum Brain Mapp. 2019;40:2800–12.
Fuentes-Claramonte P, Martín-Subero M, Salgado-Pineda P, Alonso-Lana S, Moreno-Alcázar A, Argila-Plaza I, et al. Shared and differential default-mode related patterns of activity in an autobiographical, a self-referential and an attentional task. Plos One. 2019;14:e0209376.
Gu H, Hu Y, Chen X, He Y, Yang Y. Regional excitation-inhibition balance predicts default-mode network deactivation via functional connectivity. Neuroimage. 2019;185:388–97.
Čeko M, Gracely JL, Fitzcharles M-A, Seminowicz DA, Schweinhardt P, Bushnell MC. Is a responsive default mode network required for successful working memory task performance? J Neurosci. 2015;35:11595–605.
Huang AS, Klein DN, Leung H-C. Load-related brain activation predicts spatial working memory performance in youth aged 9–12 and is associated with executive function at earlier ages. Developmental Cogn Neurosci. 2016;17:1–9.
Satterthwaite TD, Wolf DH, Erus G, Ruparel K, Elliott MA, Gennatas ED, et al. Functional maturation of the executive system during adolescence. J Neurosci. 2013;33:16249–61.
Breukelaar IA, Erlinger M, Harris A, Boyce P, Hazell P, Grieve SM, et al. Investigating the neural basis of cognitive control dysfunction in mood disorders. Bipolar Disord. 2020;22:286–95.
Gärtner M, Ghisu ME, Scheidegger M, Bönke L, Fan Y, Stippl A, et al. Aberrant working memory processing in major depression: evidence from multivoxel pattern classification. Neuropsychopharmacology. 2018;43:1972–79.
Rodríguez-Cano E, Sarró S, Monté G, Maristany T, Salvador R, McKenna P, et al. Evidence for structural and functional abnormality in the subgenual anterior cingulate cortex in major depressive disorder. Psychol Med. 2014;44:3263.
Alonso-Lana S, Goikolea JM, Bonnin CM, Sarró S, Segura B, Amann BL, et al. Structural and functional brain correlates of cognitive impairment in euthymic patients with bipolar disorder. PloS One. 2016;11:e0158867.
Rose EJ, Simonotto E, Ebmeier KP. Limbic over-activity in depression during preserved performance on the n-back task. Neuroimage. 2006;29:203–15.
Fernández-Corcuera P, Salvador R, Monté GC, Sarró SS, Goikolea JM, Amann B, et al. Bipolar depressed patients show both failure to activate and failure to de-activate during performance of a working memory task. J Affect Disord. 2013;148:170–78.
Rodríguez‐Cano E, Alonso‐Lana S, Sarró S, Fernández‐Corcuera P, Goikolea JM, Vieta E, et al. Differential failure to deactivate the default mode network in unipolar and bipolar depression. Bipolar Disord. 2017;19:386–95.
Pomarol-Clotet E, Alonso-Lana S, Moro N, Sarro S, Bonnin MC, Goikolea JM, et al. Brain functional changes across the different phases of bipolar disorder. Br J Psychiatry. 2015;206:136–44.
Pomarol-Clotet E, Moro N, Sarró S, Goikolea JM, Vieta E, Amann B, et al. Failure of de-activation in the medial frontal cortex in mania: Evidence for default mode network dysfunction in the disorder. World J Biol Psychiatry. 2012;13:616–26.
Bartova L, Meyer BM, Diers K, Rabl U, Scharinger C, Popovic A, et al. Reduced default mode network suppression during a working memory task in remitted major depression. J Psychiatr Res. 2015;64:9–18.
Alonso-Lana S, Valentí M, Romaguera A, Sarri C, Sarró S, Rodríguez-Martínez A, et al. Brain functional changes in first-degree relatives of patients with bipolar disorder: evidence for default mode network dysfunction. Psychol Med. 2016;46:2513.
Meyer BM, Rabl U, Huemer J, Bartova L, Kalcher K, Provenzano J, et al. Prefrontal networks dynamically related to recovery from major depressive disorder: a longitudinal pharmacological fMRI study. Transl Psychiatry. 2019;9:1–10.
Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–88.
Axelson D, Birmaher BJ, Brent D, Wassick S, Hoover C, Bridge J, et al. A preliminary study of the Kiddie schedule for affective disorders and schizophrenia for school-age children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol. 2003;13:463–70.
Angold A, Costello EJ, Messer SC, Pickles A. Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. Int J Methods Psychiatr Res. 1995;5:237–49.
Gerson AC, Gerring JP, Freund L, Joshi PT, Capozzoli J, Brady K, et al. The Children’s Affective Lability Scale: a psychometric evaluation of reliability. Psychiatry Res. 1996;65:189–98.
Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. J Am Acad Child Adolesc Psychiatry. 1999;38:1230–6.
Ladouceur CD, Silk JS, Dahl RE, Ostapenko L, Kronhaus DM, Phillips ML. Fearful faces influence attentional control processes in anxious youth and adults. Emotion. 2009;9:855–64.
Bertocci M, Bebko G, Dwojak A, Iyengar S, Ladouceur C, Fournier J, et al. Longitudinal relationships among activity in attention redirection neural circuitry and symptom severity in youth. Biol Psychiatry: Cogn Neurosci Neuroimaging. 2017;2:336–45.
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–39.
McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61:1277–86.
Storey JD. A direct approach to false discovery rates. J R Stat Soc Ser B-Stat Methodol. 2002;64:479–98.
Storey JD, Taylor JE, Siegmund D. Strong control, conservative point estimation and simultaneous conservative consistency of false discovery rates: a unified approach. J R Stat Soc Ser B-Stat Methodol. 2004;66:187–205.
Rogers RD, Andrews T, Grasby P, Brooks D, Robbins T. Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. J Cogn Neurosci. 2000;12:142–62.
Packard MG. Glutamate infused posttraining into the hippocampus or caudate-putamen differentially strengthens place and response learning. Proc Natl Acad Sci USA. 1999;96:12881–86.
De Simoni S, Jenkins PO, Bourke NJ, Fleminger JJ, Hellyer PJ, Jolly AE, et al. Altered caudate connectivity is associated with executive dysfunction after traumatic brain injury. Brain. 2018;141:148–64.
Stoffers D, Altena E, van der Werf YD, Sanz-Arigita EJ, Voorn TA, Astill RG, et al. The caudate: a key node in the neuronal network imbalance of insomnia? Brain. 2014;137:610–20.
Apps MA, Lockwood PL, Balsters JH. The role of the midcingulate cortex in monitoring others’ decisions. Front Neurosci. 2013;7:251.
Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–67.
Hoffstaedter F, Grefkes C, Caspers S, Roski C, Palomero‐Gallagher N, Laird AR, et al. The role of anterior midcingulate cortex in cognitive motor control: evidence from functional connectivity analyses. Hum Brain Mapp. 2014;35:2741–53.
Author information
Authors and Affiliations
Contributions
JCF, MB, and MLP conceived the analytic plan, conducted or interpreted the statistical analyses, and drafted the manuscript. MLP and BB were the principal investigators and oversaw the project. Additionally, CDL, AV, JPLS, SI aided substantially in elements of the design or interpretation of the results. LB, KM, and HA-W made substantial contributions to the acquisition of the data. All authors made significant contributions to the manuscript, and all approved the final version.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Fournier, J.C., Bertocci, M., Ladouceur, C.D. et al. Neural function during emotion regulation and future depressive symptoms in youth at risk for affective disorders. Neuropsychopharmacol. 46, 1340–1347 (2021). https://doi.org/10.1038/s41386-021-01001-w
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41386-021-01001-w
This article is cited by
-
Lifetime depression and mania/hypomania risk predicted by neural markers in three independent young adult samples during working memory and emotional regulation
Molecular Psychiatry (2025)
-
The promise of infant MRI in psychiatry: toward a framework for neural network measures in early emotional and behavioral risk identification and new intervention targets
Molecular Psychiatry (2025)
-
Alterations in functional connectivity in the salience network shared by depressive symptoms and smartphone overuse
Scientific Reports (2024)
-
Altered patterns of central executive, default mode and salience network activity and connectivity are associated with current and future depression risk in two independent young adult samples
Molecular Psychiatry (2023)