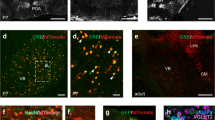
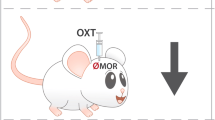
Abstract
Both the noradrenergic and galaninergic systems have been implicated in stress-related neuropsychiatric disorders, and these two neuromodulators are co-released from the stress-responsive locus coeruleus (LC); however, the individual contributions of LC-derived norepinephrine (NE) and galanin to behavioral stress responses are unclear. Here we aimed to disentangle the functional roles of co-released NE and galanin in stress-induced behavior. We used foot shock, optogenetics, and behavioral pharmacology in wild-type (WT) mice and mice lacking either NE (Dbh−/−) or galanin (GalcKO-Dbh) specifically in noradrenergic neurons to isolate the roles of these co-transmitters in regulating anxiety-like behavior in the elevated zero maze (EZM) either immediately or 24 h following stress. Foot shock and optogenetic LC stimulation produced immediate anxiety-like behavior in WT mice, and the effects of foot shock persisted for 24 h. NE-deficient mice were resistant to the anxiogenic effects of acute stress and optogenetic LC stimulation, while mice lacking noradrenergic-derived galanin displayed typical increases in anxiety-like behavior. However, when tested 24 h after foot shock, both Dbh−/− and GalcKO-Dbh mice lacked normal expression of anxiety-like behavior. Pharmacological rescue of NE, but not galanin, in knockout mice during EZM testing was anxiogenic. In contrast, restoring galanin, but not NE, signaling during foot shock normalized stress-induced anxiety-like behavior 24 h later. These results indicate that NE and noradrenergic-derived galanin play complementary, but distinguishable roles in behavioral responses to stress. NE is required for the expression of acute stress-induced anxiety, while noradrenergic-derived galanin mediates the development of more persistent responses following a stressor.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25.
Kruijshaar MK, B J, Vos T, de Graaf R, Spijker J, Andrews G. Lifetime prevalence estimates of major depression—an indirect estimation method and a quantification of recall bias. Eur J Epidemiol. 2005;20:103–11.
Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–27.
Melander T, Hokfelt T, Rokaeus A, Cuello A, Oertel W, Verhofstad A, et al. Coexistence of Galanin-like lmmunoreactivity with Catecholamines, 5-Hydroxytryptamine, GABA and Neuropeptides in the Rat CNS. J Neurosci. 1986;6:3640–54.
Skofitsch G, Jacobowitz D. Immunohistochemical mapping of galanin-like neurons in the rat central nervous system. Peptides. 1985;6:509–46.
Perez SE, Wynick D, Steiner RA, Mufson EJ. Distribution of galaninergic immunoreactivity in the brain of the mouse. J Comp Neurol. 2001;434:158–85.
Chan-Palay V, Jentsch B, Lang W, Hochli M, Asan E. Distribution of neuropeptide Y, C-terminal flanking peptide of NPY and galanin coexistence with catecholamine in the locus coeruleus of normal human, Alzheimer’s dementia and Parkinson’s disease brains. Dement Geriatr Cogn Disord. 1990;1:18–31.
Le Maitre E, Barde SS, Palkovits M, Diaz-Heijtz R, Hokfelt TG. Distinct features of neurotransmitter systems in the human brain with focus on the galanin system in locus coeruleus and dorsal raphe. Proc Natl Acad Sci USA. 2013;110:E536–45.
Holets VR, Hokfelt T, Rokaeus A, Terenius L, Goldstein M. Locus coeruleus neurons in the rat containing neuropeptide Y, tyrosine hydroxylase or galanin and their efferent projections to the spinal cord, cerebral cortex and hypothalamus. Neuroscience. 1988;24:893–906.
Karlsson RM, Holmes A. Galanin as a modulator of anxiety and depression and a therapeutic target for affective disease. Amino Acids. 2006;31:231–9.
Hokfelt T, Barde S, Xu ZD, Kuteeva E, Ruegg J, Le Maitre E, et al. Neuropeptide and small transmitter coexistence: fundamental studies and relevance to mental illness. Front Neural Circuits. 2018;12:106.
Weinshenker D, Holmes PV. Regulation of neurological and neuropsychiatric phenotypes by locus coeruleus-derived galanin. Brain Res. 2016;1641:320–37.
Borodovitsyna O, Joshi N, Chandler D. Persistent stress-induced neuroplastic changes in the locus coeruleus/norepinephrine system. Neural Plast. 2018;2018:1892570.
Valentino RJ, Van, Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharm. 2008;583:194–203.
Lang R, Gundlach AL, Holmes FE, Hobson SA, Wynick D, Hokfelt T, et al. Physiology, signaling, and pharmacology of galanin peptides and receptors: three decades of emerging diversity. Pharm Rev. 2015;67:118–75.
Bartfai T, Iverfeldt K, Fisone G, Serfozo P. Regulation of the release of coexisting neurotransmitters. Ann Rev Pharm Toxicol. 1988;28:285–310.
Sciolino NR, Holmes PV. Exercise offers anxiolytic potential: a role for stress and brain noradrenergic-galaninergic mechanisms. Neurosci Biobehav Rev. 2012;36:1965–84.
Tillage RP, Sciolino NR, Plummer NW, Lustberg D, Liles LC, Hsiang M, et al. Elimination of galanin synthesis in noradrenergic neurons reduces galanin in select brain areas and promotes active coping behaviors. Brain Struct Funct. 2020;225:785–803.
McCall JG, Siuda ER, Bhatti DL, Lawson LA, McElligott ZA, Stuber GD, et al. Locus coeruleus to basolateral amygdala noradrenergic projections promote anxiety-like behavior. Elife. 2017;6:e18247.
Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature. 1995;374:643–46.
Lustberg D, Iannitelli AF, Tillage RP, Pruitt M, Liles LC, Weinshenker D. Central norepinephrine transmission is required for stress-induced repetitive behavior in two rodent models of obsessive-compulsive disorder. Psychopharmacol (Berl). 2020;237:1973–87.
Schank JR, Liles LC, Weinshenker D. Norepinephrine signaling through β-adrenergic receptors is critical for expression of cocaine-induced anxiety. Biol Psychiatry. 2008;63:1007–12.
Marino MD, Bourdelat-Parks BN, Cameron Liles L, Weinshenker D. Genetic reduction of noradrenergic function alters social memory and reduces aggression in mice. Behav Brain Res. 2005;161:197–203.
Thomas SA, Marck BT, Palmiter RD, Matsumoto AM. Restoration of norepinephrine and reversal of phenotypes in mice lacking dopamine beta-hydroxylase. J Neurochem. 1998;70:2468–76.
Thomas SA, Palmiter RD. Disruption of the dopamine p-hydroxylase gene in mice suggests roles for norepinephrine in motor function, learning, and memory. Behav Neurosci. 1997;111:579–89.
Szot P, Weinshenker D, White S, Robbins C, Rust N, Schwartzkroin P, et al. Norepinephrine-deficient mice have increased susceptibility to seizure-inducing stimuli. J Neurosci. 1999;19:10985–92.
Gaval-Cruz M, Liles LC, Iuvone PM, Weinshenker D. Chronic inhibition of dopamine beta-hydroxylase facilitates behavioral responses to cocaine in mice. PLoS One. 2012;7:e50583.
Tillage RP, Wilson GE, Liles LC, Holmes PV, Weinshenker D. Chronic environmental or genetic elevation of galanin in noradrenergic neurons confers stress resilience in mice. J Neurosci. 2020;40:7464–74.
McCall JG, Al-Hasani R, Siuda ER, Hong DY, Norris AJ, Ford CP, et al. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron. 2015;87:605–20.
Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacol (Berl). 1994;116:56–64.
Bartfai T, Wang MW. Positive allosteric modulators to peptide GPCRs: a promising class of drugs. Acta Pharm Sin. 2013;34:880–5.
Rommelfanger KS, Edwards GL, Freeman KG, Liles LC, Miller GW, Weinshenker D. Norepinephrine loss produces more profound motor deficits than MPTP treatment in mice. Proc Natl Acad Sci USA. 2007;104:13804–9.
Murchison CF, Zhang XY, Zhang WP, Ouyang M, Lee A, Thomas SA. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117:131–43.
Lustberg D, Tillage RP, Bai Y, Pruitt M, Liles LC, Weinshenker D. Noradrenergic circuits in the forebrain control affective responses to novelty. Psychopharmacol (Berl). 2020;237:3337–55.
Cryan JF, O’Leary OF, Jin SH, Friedland JC, Ouyang M, Hirsch BR, et al. Norepinephrine-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors. Proc Natl Acad Sci USA. 2004;101:8186–91.
Sanders JD, Szot P, Weinshenker D, Happe HK, Bylund DB, Murrin LC. Analysis of brain adrenergic receptors in dopamine-beta-hydroxylase knockout mice. Brain Res. 2006;1109:45–53.
Giustino TF, Ramanathan KR, Totty MS, Miles OW, Maren S. Locus coeruleus norepinephrine drives stress-induced increases in basolateral amygdala firing and impairs extinction learning. J Neurosci. 2020;40:907–16.
Giustino TF, Seemann JR, Acca GM, Goode TD, Fitzgerald PJ, Maren S. Beta-adrenoceptor blockade in the basolateral amygdala, but not the medial prefrontal cortex, rescues the immediate extinction deficit. Neuropsychopharmacology. 2017;42:2537–44.
Llorca-Torralba M, Suarez-Pereira I, Bravo L, Camarena-Delgado C, Garcia-Partida JA, Mico JA, et al. Chemogenetic silencing of the locus coeruleus-basolateral amygdala pathway abolishes pain-induced anxiety and enhanced aversive learning in rats. Biol Psychiatry. 2019;85:1021–35.
Nava N, Treccani G, Alabsi A, Kaastrup Mueller H, Elfving B, Popoli M, et al. Temporal dynamics of acute stress-induced dendritic remodeling in medial prefrontal cortex and the protective effect of desipramine. Cereb Cortex. 2017;27:694–705.
Musazzi L, Tornese P, Sala N, Popoli M. What acute stress protocols can tell us about PTSD and stress-related neuropsychiatric disorders. Front Pharm. 2018;9:758.
Sciolino NR, Smith JM, Stranahan AM, Freeman KG, Edwards GL, Weinshenker D, et al. Galanin mediates features of neural and behavioral stress resilience afforded by exercise. Neuropharmacology. 2015;89:255–64.
Myers B, Scheimann JR, Franco-Villanueva A, Herman JP. Ascending mechanisms of stress integration: Implications for brainstem regulation of neuroendocrine and behavioral stress responses. Neurosci Biobehav Rev. 2017;74:366–75.
Levin MC, Sawchenko PE, Howe PR, Bloom SR, Polak JM. Organization of galanin-immunoreactive inputs to the paraventricular nucleus with special reference to their relationship to catecholaminergic afferents. J Comp Neurol. 1987;261:562–82.
Soares J, Holmes PV, Renner KJ, Edwards GL, Bunnell BN, Dishman RK. Brain noradrenergic responses to footshock after chronic activity-wheel running. Behav Neurosci. 1999;113:558–66.
Galvez R, Mesches MH, McGaugh JL. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiol Learn Mem. 1996;66:253–7.
Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–33.
Rajarao SJ, Platt B, Sukoff SJ, Lin Q, Bender CN, Nieuwenhuijsen BW, et al. Anxiolytic-like activity of the non-selective galanin receptor agonist, galnon. Neuropeptides. 2007;41:307–20.
Florén A, Sollenberg U, Lundström L, Zorko M, Stojan J, Budihna M, et al. Multiple interaction sites of galnon trigger its biological effects. Neuropeptides. 2005;39:547–58.
Webling KE, Runesson J, Bartfai T, Langel U. Galanin receptors and ligands. Front Endocrinol (Lausanne). 2012;3:146.
Pomrenze MB, Giovanetti SM, Maiya R, Gordon AG, Kreeger LJ, Messing RO. Dissecting the roles of GABA and neuropeptides from rat central amygdala CRF neurons in anxiety and fear learning. Cell Rep. 2019;29:13–21 e4.
Hokfelt T, Broberger C, Diez M, Xu ZQ, Shi T, Kopp J, et al. Galanin and NPY, two peptides with multiple putative roles in the nervous system. Horm Metab Res. 1999;31:330–4.
Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–8.
Acknowledgements
We thank C. Strauss for helpful editing of this manuscript.
Author information
Authors and Affiliations
Contributions
RPT and DW conceived, designed, and supervised the project. RNAscope in situ hybridization was performed by SF. Immunohistochemistry experiments were performed by RPT and DL. All behavioral experiments were performed and analyzed by RPT with assistance from KEM. Pharmacological experiments were performed by RPT with assistance from DW. Mouse husbandry and genotyping were performed by LCL. RPT and DW wrote the manuscript with input from co-authors.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Tillage, R.P., Foster, S.L., Lustberg, D. et al. Co-released norepinephrine and galanin act on different timescales to promote stress-induced anxiety-like behavior. Neuropsychopharmacol. 46, 1535–1543 (2021). https://doi.org/10.1038/s41386-021-01011-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-021-01011-8
This article is cited by
-
Disentangling the effects of corticotrophin releasing factor and GABA release from the bed nucleus of the stria terminalis on ethanol self-administration in mice
Neuropsychopharmacology (2025)
-
Dexmedetomidine elicits a prolonged anxiolytic effect by inhibiting adrenergic neurons in the locus coeruleus in mice
Translational Psychiatry (2025)
-
Maternal separation disrupts noradrenergic control of adult coping behaviors
Neuropsychopharmacology (2025)
-
Unraveling the functional complexity of the locus coeruleus-norepinephrine system: insights from molecular anatomy to neurodynamic modeling
Cognitive Neurodynamics (2025)
-
A single-vector intersectional AAV strategy for interrogating cellular diversity and brain function
Nature Neuroscience (2024)