Abstract
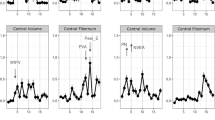
Depression is a chronic and debilitating condition that often emerges during adolescence, a period of significant brain maturation. Few studies, however, have examined how mechanisms of neuroplasticity, including myelination, are affected by adolescent-onset depression. Here, we used multimodal MR imaging to characterize myelin, indexed by R1, in white matter tracts previously associated with depression and compare 48 adolescents with lifetime depression (45 with current depression, 3 remitted) and 35 healthy controls in R1. Compared to healthy controls, R1 was higher in adolescents with lifetime depression in the uncinate fasciculus and corpus callosum genu (all βs > 0.42; all ps < 0.037). Sex significantly moderated the association between depression and R1 in the left uncinate fasciculus and corpus callosum genu (all βs > 0.86; all ps < 0.02), such that depressed female adolescents had significantly higher R1 in these tracts than did healthy female adolescents (all βs > 0.82; all ps < 0.0012). In contrast, depressed and non-depressed male adolescents did not differ in R1 in these tracts (all ps > 0.32). While fractional anisotropy (FA), a commonly examined measure of white matter organization based on diffusion-weighted MRI, in the left uncinate was positively associated with lifetime depression in our sample (β = 0.56; p = 0.016), we found no evidence of sex-specific effects of depression in FA. Our results suggest that R1 is more sensitive to sex-specific effects of depression than FA, particularly in female adolescents. Given evidence that myelin inhibits synapse formation and reduces brain plasticity, our findings implicate experience-driven regional myelination as a mechanism underlying depression during periods of significant neural maturation such as adolescence.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Code availability
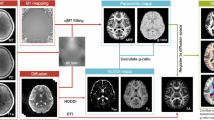
Code for preprocessing the quantitative MRI data and estimating T1 per voxel can be found here: https://github.com/cni/t1fit (t1fit_unwarp.py). Code for quality assurance, qT1 data extraction, and visualization of quantitative MRI data can be found here: https://github.com/lucindasisk/TIGER_qT1_Tracts. Code for statistical modeling can be found here: https://github.com/tiffanycheingho/TIGER.
References
Breslau J, Gilman SE, Stein BD, Ruder T, Gmelin T, Miller E. Sex differences in recent first-onset depression in an epidemiological sample of adolescents. Transl Psychiatry. 2017;7:e1139.
Leone M, Kuja-Halkola R, Leval A, D’Onofrio BM, Larsson H, Lichtenstein P, et al. Association of youth depression with subsequent somatic diseases and premature death. JAMA Psychiatry. 2021;78:302–10.
Naicker K, Galambos NL, Zeng Y, Senthilselvan A, Colman I. Social, demographic, and health outcomes in the 10 years following adolescent depression. J Adolesc Health. 2013;52:533–8.
Young KM, Psachoulia K, Tripathi RB, Dunn SJ, Cossell L, Attwell D, et al. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77:873–85.
Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344:6183.
Long P, Corfas G. Dynamic regulation of myelination in health and disease. JAMA Psychiatry. 2014;71:1296–7.
Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage. 2013;73:239–54.
LeWinn KZ, Connolly CG, Wu J, Drahos M, Hoeft F, Ho TC, et al. White matter correlates of adolescent depression: structural evidence for frontolimbic disconnectivity. J Am Acad Child Adolesc Psychiatry. 2014;53:899–909.
Van Velzen LS, Kelly S, Isaev D, Aleman A, Aftanas LI, Bauer J, et al. White matter disturbances in major depressive disorder: a coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Mol Psychiatry. 2019;25:1511–25.
Aghajani M, Veer IM, van Lang ND, Meens PH, van den Bulk BG, Rombouts SA, et al. Altered white-matter architecture in treatment-naive adolescents with clinical depression. Psychological Med. 2014;44:2287–98.
Heath F, Hurley SA, Johansen-Berg H, Sampaio-Baptista C. Advances in noninvasive myelin imaging. Dev Neurobiol. 2017;78:136–51.
Stüber C, Morawski M, Schäfer A, Labadie C, Wähnert M, Leuze C. Myelin and iron concentration in the human brain: a quantitative study of MRI contrast. Neuroimage. 2014;93:95–106.
Mottershead JP, Schmierer K, Clemence M, Thornton JS, Scaravilli F, Barker GJ. High field MRI correlates of myelin content and axonal density in multiple sclerosis–a post-mortem study of the spinal cord. J Neurol. 2003;250:1293–301.
Schmierer K, Tozer DJ, Scaravilli F, Altmann DR, Barker GJ, Tofts PS, et al. Quantitative magnetization transfer imaging in postmortem multiple sclerosis brain. J Magn Reson Imaging. 2007;26:41–51.
Schmierer K, Wheeler-Kingshott CA, Tozer DJ, Boulby PA, Parkes HG, Yousry TA, et al. Quantitative magnetic resonance of postmortem multiple sclerosis brain before and after fixation. Magn Reson Med. 2008;59:268–77.
Lutti A, Dick F, Sereno MI, Weiskopf N. Using high-resolution quantitative mapping of R1 as an index of cortical myelination. Neuroimage. 2013;93:176–88.
Sacchet MD, Gotlib IH. Myelination of the brain in Major Depressive Disorder: an in vivo quantitative magnetic resonance imaging study. Sci Rep. 2017;7:2200.
Kumar A, Gupta RC, Albert Thomas M, Alger J, Wyckoff N, Hwang S. Biophysical changes in normal-appearing white matter and subcortical nuclei in late-life major depression detected using magnetization transfer. Psychiatry Res. 2004;130:131–40.
Gunning-Dixon FM, Hoptman MJ, Lim KO, Murphy CF, Klimstra S, Latoussakis V. Macromolecular white matter abnormalities in geriatric depression: a magnetization transfer imaging study. Am J Geriatr Psychiatry. 2008;16:255–62.
Yeatman JD, Wandell BA, Mezer AA. Lifespan maturation and degeneration of human brain white matter. Nat Commun. 2014;5:4932.
Cerghet M, Skoff RP, Bessert D, Zhang Z, Mullins C, Ghandour MS. Proliferation and Death of Oligodendrocytes and Myelin Proteins Are Differentially Regulated in Male and Female Rodents. J Neurosci. 2006;26:1439–47.
Yates MA, Juraska JM. Pubertal ovarian hormone exposure reduces the number of myelinated axons in the splenium of the rat corpus callosum. Exp Neurol. 2008;209:284–7.
Walker JC, Teresi GI, Weisenburger RL, Segarra JR, Ojha A, Kulla A. Study Protocol for Teen Inflammation Glutamate Emotion Research (TIGER). Front Hum Neurosci. 2020;14:585512.
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;208:19.
Wu H, Dougherty RF, Kerr AB, Zhu K, Middione MJ. Fast T1 mapping using slice‐shuffled simultaneous multi‐slice inversion recovery EPI. Int Soc Magn Reson Med. 2015;1057:440.
Ho TC, King LS, Leong JK, Colich NL, Humphreys KL, Ordaz SJ, et al. Effects of sensitivity to life stress on uncinate fasciculus segments in early adolescence. Soc Cogn Affect Neurosci. 2017;12:1460–9.
Ho TC, Colich NL, Sisk LM, Oskirko K, Jo B, Gotlib IH. Sex differences in the effects of gonadal hormones on white matter microstructure development in adolescence. Dev Cogn Neurosci. 2020;42:100773.
Kircanski K, Sisk LM, Ho TC, Humphreys KL, King LS, Colich NL, et al. Early life stress, cortisol, frontolimbic connectivity, and depressive symptoms during puberty. Dev Psychopathol. 2019;31:1011–22.
Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8.
Reynolds WM. Reynolds Adolescent Depression Scale. In: Hersen M, Segal DM, Hilsenroth M, eds. Comprehensive handbook of psychological assessment, Volume 2: personality assessment and psychopathology. 2nd ed. New York, NY: John Wiley & Sons; 2004. p. 224–36.
Seitz J, Cetin-Karayumak S, Lyall A, Pasternak O, Baxi M, Vangel M, et al. Investigating Sexual Dimorphism of Human White Matter in a Harmonized, Multisite Diffusion Magnetic Resonance Imaging Study. Cereb Cortex. 2021;31:201–12.
Stevens B, Porta S, Haak LL, Gallo V, Fields RD. Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron. 2002;36:855–68.
Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, et al. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49:823–32.
Chetty S, Friedman AR, Taravosh-Lahn K, Kirby ED, Mirescu C, Guo F, et al. Stress and glucocorticoids promote oligodendrogenesis in the adult hippocampus. Mol Psychiatry. 2014;19:1275–83.
Chao LL, Tosun D, Woodward SH, Kaufer D, Neylan TC. Preliminary Evidence of Increased Hippocampal Myelin Content in Veterans with Posttraumatic Stress Disorder. Front Behav Neurosci. 2015;9:333.
Bandtlow C, Zachleder T, Schwab ME. Oligodendrocytes arrest neurite growth by contact inhibition. J Neurosci. 1990;10:3837–48.
Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–9.
McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–6.
McGee AW, Strittmatter SM. The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci. 2003;26:193–8.
Marin-Husstege M, Muggironi M, Raban D, Skoff RP, Casaccia-Bonnefil P. Oligodendrocyte progenitor proliferation and maturation is differentially regulated by male and female sex steroid hormones. Dev Neurosci. 2004;26:245–54.
Koss WA, Lloyd MM, Sadowski RN, Wise LM, Juraska JM. Gonadectomy before puberty increases the number of neurons and glia in the medial prefrontal cortex of female, but not male, rats. Dev Psychobiol. 2015;57:305–12.
Vijayakumar N, Op de Macks Z, Shirtcliff EA, Pfeifer JH. Puberty and the human brain: insights into adolescent development. Neurosci Biobehav Rev. 2018;92:417–36.
Lindqvist D, Simon NM, Wolkowitz OM. Is Depression Associated With Accelerated Aging? Mechanisms and Implications. In: Quevedo J, Carvalho A, Zarate CA, eds. Neurobiology of Depression, 1st ed. Cambridge, MA: Academic Press; 2019. p. 207–29.
Humphreys KL, Sisk LM, Manczak EM, Lin J, Gotlib IH. Depressive Symptoms Predict Change in Telomere Length and Mitochondrial DNA Copy Number Across Adolescence. J Am Acad Child Adolesc Psychiatry. 2020;59:1364–70.
Han LKM, Dinga R, Hahn T, Ching CRK, Eyler LT, Aftanas L, et al. Brain aging in major depressive disorder: results from the ENIGMA major depressive disorder working group. Mol Psychiatry. 2020. https://doi.org/10.1038/s41380-020-0754-0.
Yeatman JD, Wandell BA, Mezer AA. Lifespan maturation and degeneration of human brain white matter. Nat Commun. 2014;5:4932.
Colich NL, Rosen ML, Williams ES, McLaughlin KA. Biological aging in childhood and adolescence following experiences of threat and deprivation: a systematic review and meta-analysis. Psychol Bull. 2020;146:721–64.
Acknowledgements
We thank Alexess Sosa, Anna Cichocki, Amar Ojha, Holly Pham, and Kira Oskirko for assistance with data collection and organization. We also thank Lucy King and Jaclyn Schwartz for helpful comments during the preparation of this paper. Finally, we wish to thank the participants and their families for contributing to this study.
Funding
This research was supported in part by the National Institutes of Health (K01MH117442 to TCH and R37MH101495 to IHG), the Ray and Dagmar Dolby Family Fund (TCH), Stanford Maternal & Child Health Research Institute (TCH), Stanford Center for Cognitive and Neurobiological Imaging University (TCH), Stanford Precision Health and Integrated Diagnostics (IHG and TCH), the Klingenstein Third Generation Foundation (Child and Adolescent Depression Award to TCH), the National Science Foundation (Graduate Research Fellowship Award NSF DGE-1752134 to LMS), and Stanford Bio-X (Undergraduate Summer Research Program to AK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Science Foundation.
Author information
Authors and Affiliations
Contributions
TCH conceived and obtained funding for the study; TCH and LMS designed the study and drafted the first version of the paper; TCH, LMS, AK, GT, MMH, and HW contributed to the acquisition and analysis of the data; TCH, LMS, and IHG interpreted the data; all authors reviewed the paper and critically revised it for important intellectual content. All authors agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
All authors report no biomedical competing interest. The funding agencies played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ho, T.C., Sisk, L.M., Kulla, A. et al. Sex differences in myelin content of white matter tracts in adolescents with depression. Neuropsychopharmacol. 46, 2295–2303 (2021). https://doi.org/10.1038/s41386-021-01078-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-021-01078-3
This article is cited by
-
Oligodendrocyte lineage cells dysfunction in depression: early life stress, adolescent vulnerability and the emerging role of lipid metabolism
Translational Psychiatry (2025)
-
Classification of major depressive disorder using vertex-wise brain sulcal depth, curvature, and thickness with a deep and a shallow learning model
Molecular Psychiatry (2025)
-
Understanding the phenotypic variability in Niemann-Pick disease type C (NPC): a need for precision medicine
npj Genomic Medicine (2023)
-
Brain-based Sex Differences in Depression: A Systematic Review of Neuroimaging Studies
Brain Imaging and Behavior (2023)