Abstract
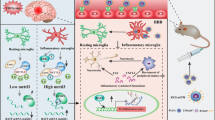
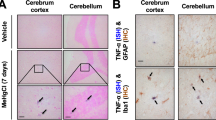
Methamphetamine (Meth) is a powerful illicit psychostimulant, widely used for recreational purposes. Besides disrupting the monoaminergic system and promoting oxidative brain damage, Meth also causes neuroinflammation, contributing to synaptic dysfunction and behavioral deficits. Aberrant activation of microglia, the largest myeloid cell population in the brain, is a common feature in neurological disorders triggered by neuroinflammation. In this study, we investigated the mechanisms underlying the aberrant activation of microglia elicited by Meth in the adult mouse brain. We found that binge Meth exposure caused microgliosis and disrupted risk assessment behavior (a feature that usually occurs in individuals who abuse Meth), both of which required astrocyte-to-microglia crosstalk. Mechanistically, Meth triggered a detrimental increase of glutamate exocytosis from astrocytes (in a process dependent on TNF production and calcium mobilization), promoting microglial expansion and reactivity. Ablating TNF production, or suppressing astrocytic calcium mobilization, prevented Meth-elicited microglia reactivity and re-established risk assessment behavior as tested by elevated plus maze (EPM). Overall, our data indicate that glial crosstalk is critical to relay alterations caused by acute Meth exposure.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Thanos PK, Kim R, Delis F, Ananth M, Chachati G, Rocco MJ, et al. Chronic methamphetamine effects on brain structure and function in rats. PLoS One. 2016;11:e0155457.
Chang X, Sun Y, Zhang Y, Muhai J, Lu L, Shi J. A review of risk factors for methamphetamine-related psychiatric symptoms. Front Psychiatry. 2018;9:603.
Cadet JL, Bisagno V, Milroy CM. Neuropathology of substance use disorders. Acta Neuropathol. 2014;127:91–107.
Moszczynska A, Callan SP. Molecular, behavioral, and physiological consequences of methamphetamine neurotoxicity: implications for treatment. J Pharm Exp Ther. 2017;362:474–88.
Northrop NA, Halpin LE, Yamamoto BK. Peripheral ammonia and blood brain barrier structure and function after methamphetamine. Neuropharmacology. 2016;107:18–26.
Shaerzadeh F, Streit WJ, Heysieattalab S, Khoshbouei H. Methamphetamine neurotoxicity, microglia, and neuroinflammation. J Neuroinflammation. 2018;15:341.
Yamamoto BK, Raudensky J. The role of oxidative stress, metabolic compromise, and inflammation in neuronal injury produced by amphetamine-related drugs of abuse. J Neuroimmune Pharm. 2008;3:203–17.
Cadet JL, Bisagno V. Glial-neuronal ensembles: partners in drug addiction-associated synaptic plasticity. Front Pharm. 2014;5:204.
Miguel-Hidalgo JJ. The role of glial cells in drug abuse. Curr Drug Abus Rev. 2009;2:72–82.
Beardsley PM, Hauser KF. Glial modulators as potential treatments of psychostimulant abuse. Adv Pharm. 2014;69:1–69.
Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron 2014;81:728–39.
Tzschentke TM, Schmidt WJ. Glutamatergic mechanisms in addiction. Mol Psychiatry. 2003;8:373–82.
Bazargani N, Attwell D. Astrocyte calcium signaling: the third wave. Nat Neurosci. 2016;19:182–9.
Clark KH, Wiley CA, Bradberry CW. Psychostimulant abuse and neuroinflammation: emerging evidence of their interconnection. Neurotox Res. 2013;23:174–88.
Krasnova IN, Justinova Z, Cadet JL. Methamphetamine addiction: involvement of CREB and neuroinflammatory signaling pathways. Psychopharmacol (Berl). 2016;233:1945–62.
Salter MW, Beggs S. Sublime microglia: expanding roles for the guardians of the CNS. Cell 2014;158:15–24.
Harms AS, Lee JK, Nguyen TA, Chang J, Ruhn KM, Trevino I, et al. Regulation of microglia effector functions by tumor necrosis factor signaling. Glia. 2012;60:189–202.
Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15:300–12.
Biber K, Moller T, Boddeke E, Prinz M. Central nervous system myeloid cells as drug targets: current status and translational challenges. Nat Rev Drug Disco. 2016;15:110–24.
Stephenson J, Nutma E, van der Valk P, Amor S. Inflammation in CNS neurodegenerative diseases. Immunology. 2018;154:204–19.
Subhramanyam CS, Wang C, Hu Q, Dheen ST. Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin Cell Dev Biol. 2019;94:112–20.
Socodato R, Henriques JF, Portugal CC, Almeida TO, Tedim-Moreira J, Alves RL, et al. Daily alcohol intake triggers aberrant synaptic pruning leading to synapse loss and anxiety-like behavior. Sci Signal. 2020;13:eaba5754.
ter Horst JP, de Kloet ER, Schachinger H, Oitzl MS. Relevance of stress and female sex hormones for emotion and cognition. Cell Mol Neurobiol. 2012;32:725–35.
Li X, Zima AV, Sheikh F, Blatter LA, Chen J. Endothelin-1-induced arrhythmogenic Ca2+ signaling is abolished in atrial myocytes of inositol-1,4,5-trisphosphate(IP3)-receptor type 2-deficient mice. Circ Res. 2005;96:1274–81.
Guerra-Gomes S, Sousa N, Pinto L, Oliveira JF. Functional roles of astrocyte calcium elevations: from synapses to behavior. Front Cell Neurosci. 2018;11:427.
Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharm Exp Ther. 2004;311:1–7.
Nakajima A, Yamada K, Nagai T, Uchiyama T, Miyamoto Y, Mamiya T, et al. Role of tumor necrosis factor-alpha in methamphetamine-induced drug dependence and neurotoxicity. J Neurosci. 2004;24:2212–25.
Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60:379–407.
Galatro TF, Vainchtein ID, Brouwer N, Boddeke E, Eggen BJL. Isolation of microglia and immune infiltrates from mouse and primate central nervous system. Methods Mol Biol. 2017;1559:333–42.
Socodato R, Portugal CC, Canedo T, Rodrigues A, Almeida TO, Henriques JF, et al. Microglia dysfunction caused by the loss of rhoa disrupts neuronal physiology and leads to neurodegeneration. Cell Rep. 2020;31:107796.
Andrade EB, Magalhaes A, Puga A, Costa M, Bravo J, Portugal CC, et al. A mouse model reproducing the pathophysiology of neonatal group B streptococcal infection. Nat Commun. 2018;9:3138.
Portugal CC, Socodato R, Canedo T, Silva CM, Martins T, Coreixas VS, et al. Caveolin-1-mediated internalization of the vitamin C transporter SVCT2 in microglia triggers an inflammatory phenotype. Sci Signal. 2017;10:eaal2005.
Bortell N, Basova L, Semenova S, Fox HS, Ravasi T, Marcondes MC. Astrocyte-specific overexpressed gene signatures in response to methamphetamine exposure in vitro. J Neuroinflammation 2017;14:49.
Socodato R, Portugal CC, Domith I, Oliveira NA, Coreixas VS, Loiola EC, et al. c-Src function is necessary and sufficient for triggering microglial cell activation. Glia 2015;63:497–511.
Koenigsknecht J, Landreth G. Microglial phagocytosis of fibrillar beta-amyloid through a beta1 integrin-dependent mechanism. J Neurosci. 2004;24:9838–46.
Socodato R, Melo P, Ferraz-Nogueira JP, Portugal CC, Relvas JB. A protocol for FRET-based live-cell imaging in microglia. STAR Protocols. 2020;1:100147.
Mateus-Pinheiro A, Alves ND, Patrício P, Machado-Santos AR, Loureiro-Campos E, Silva JM, et al. AP2γ controls adult hippocampal neurogenesis and modulates cognitive, but not anxiety or depressive-like behavior. Mol Psychiatry. 2017;22:1725–34.
Ayata P, Badimon A, Strasburger HJ, Duff MK, Montgomery SE, Loh Y-HE, et al. Epigenetic regulation of brain region-specific microglia clearance activity. Nat Neurosci. 2018;21:1049–60.
Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, et al. Identification of a unique TGF-β–dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17:131–43.
Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A, et al. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity. 2019;50:253–71.e6.
Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, et al. a unique microglia type associated with restricting development of Alzheimer’s disease. Cell. 2017;169:1276–90.e17.
Najera JA, Bustamante EA, Bortell N, Morsey B, Fox HS, Ravasi T, et al. Methamphetamine abuse affects gene expression in brain-derived microglia of SIV-infected macaques to enhance inflammation and promote virus targets. BMC Immunol. 2016;17:7–7.
Savell KE, Tuscher JJ, Zipperly ME, Duke CG, Phillips RA, Bauman AJ, et al. A dopamine-induced gene expression signature regulates neuronal function and cocaine response. Sci Adv. 2020;6:eaba4221.
Astarita G, Avanesian A, Grimaldi B, Realini N, Justinova Z, Panlilio LV, et al. Methamphetamine accelerates cellular senescence through stimulation of de novo ceramide biosynthesis. PLoS One. 2015;10:e0116961.
Liddelow SA, Marsh SE, Stevens B. Microglia and astrocytes in disease: dynamic duo or partners in crime? Trends Immunol. 2020;41:820–35.
Socodato R, Portugal CC, Canedo T, Domith I, Oliveira NA, Paes-de-Carvalho R, et al. c-Src deactivation by the polyphenol 3-O-caffeoylquinic acid abrogates reactive oxygen species-mediated glutamate release from microglia and neuronal excitotoxicity. Free Radic Biol Med. 2015;79:45–55.
Goncalves J, Martins T, Ferreira R, Milhazes N, Borges F, Ribeiro CF, et al. Methamphetamine-induced early increase of IL-6 and TNF-alpha mRNA expression in the mouse brain. Ann NY Acad Sci. 2008;1139:103–11.
Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, et al. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–10.
Okumoto S, Looger LL, Micheva KD, Reimer RJ, Smith SJ, Frommer WB. Detection of glutamate release from neurons by genetically encoded surface-displayed FRET nanosensors. Proc Natl Acad Sci USA. 2005;102:8740–5.
Harada K, Kamiya T, Tsuboi T. Gliotransmitter release from astrocytes: functional, developmental, and pathological implications in the brain. Front Neurosci. 2015;9:499.
Parpura V, Grubisic V, Verkhratsky A. Ca(2+) sources for the exocytotic release of glutamate from astrocytes. Biochim Biophys Acta. 2011;1813:984–91.
Palmer AE, Jin C, Reed JC, Tsien RY. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc Natl Acad Sci USA. 2004;101:17404–9.
Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, et al. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19:723–33.
Verkhratsky A, Matteoli M, Parpura V, Mothet JP, Zorec R. Astrocytes as secretory cells of the central nervous system: idiosyncrasies of vesicular secretion. EMBO J. 2016;35:239–57.
Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol Rev. 2000;80:717–66.
Petravicz J, Fiacco TA, McCarthy KD. Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J Neurosci. 2008;28:4967–73.
Blank T, Prinz M. Microglia as modulators of cognition and neuropsychiatric disorders. Glia. 2013;61:62–70.
Rooney S, Sah A, Unger MS, Kharitonova M, Sartori SB, Schwarzer C, et al. Neuroinflammatory alterations in trait anxiety: modulatory effects of minocycline. Transl Psychiatry. 2020;10:256.
Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, et al. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci. 2008;28:5756–61.
Buchanan JB, Sparkman NL, Johnson RW. A neurotoxic regimen of methamphetamine exacerbates the febrile and neuroinflammatory response to a subsequent peripheral immune stimulus. J Neuroinflammation. 2010;7:82.
Loftis JM, Choi D, Hoffman W, Huckans MS. Methamphetamine causes persistent immune dysregulation: a cross-species, translational report. Neurotox Res. 2011;20:59–68.
Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69.
Coelho-Santos V, Goncalves J, Fontes-Ribeiro C, Silva AP. Prevention of methamphetamine-induced microglial cell death by TNF-alpha and IL-6 through activation of the JAK-STAT pathway. J Neuroinflammation. 2012;9:103.
Frank MG, Adhikary S, Sobesky JL, Weber MD, Watkins LR, Maier SF. The danger-associated molecular pattern HMGB1 mediates the neuroinflammatory effects of methamphetamine. Brain Behav Immun. 2016;51:99–108.
Lewitus GM, Konefal SC, Greenhalgh AD, Pribiag H, Augereau K, Stellwagen D. Microglial TNF-alpha suppresses cocaine-induced plasticity and behavioral sensitization. Neuron. 2016;90:483–91.
Escartin C, Galea E, Lakatos A, O’Callaghan JP, Petzold GC, Serrano-Pozo A, et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci. 2021;24:312–25.
Narita M, Miyatake M, Narita M, Shibasaki M, Shindo K, Nakamura A, et al. Direct evidence of astrocytic modulation in the development of rewarding effects induced by drugs of abuse. Neuropsychopharmacology. 2006;31:2476–88.
Du SH, Qiao DF, Chen CX, Chen S, Liu C, Lin Z, et al. Toll-like receptor 4 mediates methamphetamine-induced neuroinflammation through caspase-11 signaling pathway in astrocytes. Front Mol Neurosci. 2017;10:409.
Dang J, Tiwari SK, Agrawal K, Hui H, Qin Y, Rana TM. Glial cell diversity and methamphetamine-induced neuroinflammation in human cerebral organoids. Mol Psychiatry. 2021;26:1194–207.
Dong Y, Benveniste EN. Immune function of astrocytes. Glia 2001;36:180–90.
Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–45.
Rossi D. Astrocyte physiopathology: at the crossroads of intercellular networking, inflammation and cell death. Prog Neurobiol. 2015;130:86–120.
Domercq M, Brambilla L, Pilati E, Marchaland J, Volterra A, Bezzi P. P2Y1 receptor-evoked glutamate exocytosis from astrocytes: control by tumor necrosis factor-alpha and prostaglandins. J Biol Chem. 2006;281:30684–96.
Sitcheran R, Gupta P, Fisher PB, Baldwin AS. Positive and negative regulation of EAAT2 by NF-kappaB: a role for N-myc in TNFalpha-controlled repression. EMBO J. 2005;24:510–20.
Wang Z, Pekarskaya O, Bencheikh M, Chao W, Gelbard HA, Ghorpade A, et al. Reduced expression of glutamate transporter EAAT2 and impaired glutamate transport in human primary astrocytes exposed to HIV-1 or gp120. Virology. 2003;312:60–73.
Doble A. The role of excitotoxicity in neurodegenerative disease: implications for therapy. Pharm Ther. 1999;81:163–221.
Cisneros IE, Ghorpade A. Methamphetamine and HIV-1-induced neurotoxicity: role of trace amine associated receptor 1 cAMP signaling in astrocytes. Neuropharmacology. 2014;85:499–507.
Yan Y, Nitta A, Koseki T, Yamada K, Nabeshima T. Dissociable role of tumor necrosis factor alpha gene deletion in methamphetamine self-administration and cue-induced relapsing behavior in mice. Psychopharmacol. 2012;221:427–36.
Volterra A, Liaudet N, Savtchouk I. Astrocyte Ca(2)(+) signalling: an unexpected complexity. Nat Rev Neurosci. 2014;15:327–35.
Corkrum M, Covelo A, Lines J, Bellocchio L, Pisansky M, Loke K, et al. Dopamine-evoked synaptic regulation in the nucleus accumbens requires astrocyte activity. Neuron. 2020;105:1036–47 e5.
Acknowledgements
We acknowledge the support of the following i3S Scientific Platforms: Animal Facility, Cell Culture and Genotyping (CCGen), Translational Cytometry Unit (TraCy), and the assistance of Mafalda Rocha (Genomics platform) and Maria Azevedo (ALM platform) and André Maia (BioSciences screening). We also acknowledge our late colleague Rui Applelberg for kindly make TNF KO mice available to us, and the designer Maria Summavielle for her contribution in assembling the figures that illustrate this publication. RS has contributed as first author.
Funding
This work was financed by FEDER—Fundo Europeu de Desenvolvimento Regional funds through the COMPETE 2020 - Operational Programme for Competitiveness and Internationalisation (POCI), Portugal 2020, and by Portuguese funds through FCT—Fundação para a Ciência e a Tecnologia/Ministério da Ciência (FCT), Tecnologia e Ensino Superior in the framework of the project POCI-01-0145-FEDER-030647 (PTDC/SAU-TOX/30647/2017) in TS lab. FEDER Portugal (Norte-01-0145-FEDER-000008000008—Porto Neurosciences and Neurologic Disease Research Initiative at I3S, supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF); FCOMP-01-0124-FEDER-021333). CCP and RS hold employment contracts financed by national funds through FCT –in the context of the program-contract described in paragraphs 4, 5, and 6 of art. 23 of Law no. 57/2016, of August 29, as amended by Law no. 57/2017 of July 2019. TC, TOA, AFT, JB, AIS and AM were supported by FCT (SFRH/BD/117148/2016, SFRH/BD/147981/2019, 2020.07188.BD, PD/BD/135450/2017, SFRH/BD/144324/2019, and IF/00753/2014). Work in JBR lab was supported by the FCT project PTDC/ MED-NEU/31318/2017. JFO was also supported by FCT projects PTDC/MED-NEU/31417/2017 and POCI-01-0145-FEDER-016818; Bial Foundation Grants 207/14 and 037/18, by National funds, through FCT - project UIDB/50026/2020; and by the projects NORTE-01-0145-FEDER-000013 and NORTE-01-0145-FEDER-000023, supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). Funding of i3S Scientific Platforms: Advanced Light Microscopy (ALM), a member of the national infrastructure PPBI-Portuguese Platform of BioImaging (POCI-01–0145-FEDER-022122); and Genomics through GenomePT project (POCI-01-0145-FEDER-022184), supported by COMPETE 2020—Operational Programme for Competitiveness and Internationalization (POCI), Lisboa Portugal Regional Operational Programme (Lisboa2020), Algarve Portugal Regional Operational Programme (CRESC Algarve2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF), and by FCT.
Author information
Authors and Affiliations
Contributions
Conception of the work—TC, CCP, RS, JBR, TS; Acquisition, analysis, or interpretation of data for the work—TC, CCP, RS, TOA, AFT, JB, AIS, JDM, SGG, JFO, AM, JBR, TS; Drafting the work or revising it critically—TC, CCP, RS, NS, JFO, JBR, TS; Final approval of the version to be published- TC, CCP, TS. Agreement to be accountable for all aspects of the work in ensuring accuracy and integrity—CCP and TS.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Canedo, T., Portugal, C.C., Socodato, R. et al. Astrocyte-derived TNF and glutamate critically modulate microglia activation by methamphetamine. Neuropsychopharmacol. 46, 2358–2370 (2021). https://doi.org/10.1038/s41386-021-01139-7
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41386-021-01139-7
This article is cited by
-
The interaction between central and peripheral immune systems in methamphetamine use disorder: current status and future directions
Journal of Neuroinflammation (2025)
-
Beyond the gut: decoding the gut–immune–brain axis in health and disease
Cellular & Molecular Immunology (2025)
-
The effects of methylphenidate and atomoxetine on Drosophila brain at single-cell resolution and potential drug repurposing for ADHD treatment
Molecular Psychiatry (2024)
-
Nimodipine attenuates neuroinflammation and delayed apoptotic neuronal death induced by trimethyltin in the dentate gyrus of mice
Journal of Molecular Histology (2024)
-
Methamphetamine-induced encephalopathy in the absence of hyperammonemia
BMC Psychiatry (2023)