Abstract
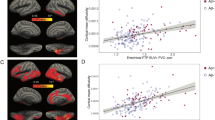
Recent evidence suggests an association between benzodiazepines (BZDs) use and lower brain amyloid load, a hallmark of AD pathophysiology. Other AD-related markers include hippocampal atrophy, but the effect of BZDs on hippocampal volume remains unclear. We aimed at 1) replicating findings on BZDs use and brain amyloid load and 2) investigating associations between BZDs use and hippocampal volume, in the MEMENTO clinical cohort of nondemented older adults with isolated memory complaint or light cognitive impairment at baseline. Total Standardized Uptake Value Ratio (SUVR) of brain amyloid load and hippocampal volume (HV) were obtained, respectively, from 18F Florbetapir positron emission tomography (PET) and magnetic resonance imaging (MRI), and compared between BZD chronic users and nonusers using multiple linear regressions adjusted for age, sex, educational level, ApoE ε4 genotype, cognitive and neuropsychiatric assessments, history of major depressive episodes and antidepressant intake. BZD users were more likely to manifest symptoms of depression, anxiety and apathy. In the MRI subgroup, BZD users were also more frequently females with low education and greater clinical impairments as assessed with the clinical dementia rating scale. Short- versus long-acting BZDs, Z-drugs versus non-Z-drugs BZDs, as well as dose and duration of BZD use, were also considered in the analyses. Total SUVR and HV were significantly lower and larger, respectively, in BZD users (n = 38 in the PET subgroup and n = 331 in the MRI subgroup) than in nonusers (n = 251 in the PET subgroup and n = 1840 in the MRI subgroup), with a medium (Cohen’s d = −0.43) and low (Cohen’s d = 0.10) effect size, respectively. Short-acting BZDs and Z-drugs were more significantly associated with larger HV. We found no effect of dose and duration of BZD use. Our results support the involvement of the GABAergic system as a potential target for blocking AD-related pathophysiology, possibly via reduction in neuronal activity and neuroinflammation. Future longitudinal studies may confirm the causal effect of BZDs to block amyloid accumulation and hippocampal atrophy.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Islam MM, Iqbal U, Walther B, Atique S, Dubey NK, Nguyen PA, et al. Benzodiazepine use and risk of dementia in the elderly population: a systematic review and meta-analysis. Neuroepidemiology. 2016;47:181–91.
Tseng L-Y, Huang S-T, Peng L-N, Chen L-K, Hsiao F-Y. Benzodiazepines, z-hypnotics, and risk of dementia: special considerations of half-lives and concomitant use. Neurotherapeutics. 2020;17:156–64.
Osler M, Jørgensen MB. Associations of benzodiazepines, Z-drugs, and other anxiolytics with subsequent dementia in patients with affective disorders: a nationwide cohort and nested case-control study. Am J Psychiatry. 2020;177:497–505.
Reyes AT, Constantino RE, Arenas RA, Bombard JN, Acupan AR. Exploring challenges in conducting E-mental health research among Asian American women. AsianPacific Isl Nurs J. 2018;3:139–53.
Quiroga C, Chaparro RE, Karlnoski R, Erasso D, Gordon M, Morgan D, et al. Effects of repetitive exposure to anesthetics and analgesics in the Tg2576 mouse Alzheimer’s model. Neurotox Res. 2014;26:414–21.
Tampellini D, Capetillo-Zarate E, Dumont M, Huang Z, Yu F, Lin MT, et al. Effects of synaptic modulation on -amyloid, synaptophysin, and memory performance in Alzheimer’s disease transgenic mice. J Neurosci. 2010;30:14299–304.
Yamamoto N, Arima H, Sugiura T, Hirate H, Kusama N, Suzuki K, et al. Midazolam inhibits the formation of amyloid fibrils and GM1 ganglioside-rich microdomains in presynaptic membranes through the gamma-aminobutyric acid A receptor. Biochem Biophys Res Commun. 2015;457:547–53.
Chung JK, Nakajima S, Shinagawa S, Plitman E, Chakravarty MM, Iwata Y, et al. Benzodiazepine use attenuates cortical β-Amyloid and is not associated with progressive cognitive decline in nondemented elderly adults: a pilot study using F18-Florbetapir positron emission tomography. Am J Geriatr Psychiatry. 2016;24:1028–39.
Desmidt T, Delrieu J, Lebouvier T, Robert G, David R, Balageas AC, et al. Benzodiazepine use and brain amyloid load in nondemented older individuals: a florbetapir PET study in the Multidomain Alzheimer Preventive Trial cohort. Neurobiol. Aging. 2019;84:61–69.
Dufouil C, Dubois B, Vellas B, Pasquier F, Blanc F, Hugon J, et al. Cognitive and imaging markers in non-demented subjects attending a memory clinic: study design and baseline findings of the MEMENTO cohort. Alzheimers Res Ther. 2017;9:67.
Wong DF, Rosenberg PB, Zhou Y, Kumar A, Raymont V, Ravert HT, et al. In vivo imaging of amyloid deposition in alzheimer disease using the radioligand 18 F-AV-45 (Flobetapir F 18). J Nucl Med. 2010;51:913–20.
Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 2012;11:669–78.
Operto G, Chupin M, Batrancourt B, Habert MO, Colliot O, Benali H, et al. CATI: a large distributed infrastructure for the neuroimaging of cohorts. Neuroinformatics. 2016;14:253–64.
Habert M-O, Marie S, Bertin H, Reynal M, Martini JB, Diallo M, et al. Optimization of brain PET imaging for a multicentre trial: the French CATI experience. EJNMMI Phys. 2016;3:6.
Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51.
Chupin M, Hammers A, Liu RSN, Colliot O, Burdett J, Bardinet E, et al. Automatic segmentation of the hippocampus and the amygdala driven by hybrid constraints: method and validation. Neuroimage. 2009;46:749–61.
Habert M-O, Bertin H, Labit M, Diallo M, Marie S, Martineau K, et al. Evaluation of amyloid status in a cohort of elderly individuals with memory complaints: validation of the method of quantification and determination of positivity thresholds. Ann Nucl Med. 2018;32:75–86.
Haider SI, Johnell K, Weitoft GR, Thorslund M, Fastbom J. The influence of educational level on polypharmacy and inappropriate drug use: a register-based study of more than 600,000 older people: polypharmacy and inappropriate drug use in the elderly. J Am Geriatr Soc. 2009;57:62–69.
Crowe SF, Stranks E. Sci-Hub | The residual medium and long-term cognitive effects of benzodiazepine use: an updated meta-analysis. Arch Clin Neuropsychol. 2017. https://doi.org/10.1093/arclin/acx120. https://sci-hubtw.hkvisa.net/.
Conejero I, Dubois J, Gutierrez LA, Delrieu J, Arbus C, Garcia M, et al. Amyloid burden and depressive symptom trajectories in older adults at risk of developing cognitive decline. J Clin Psychiatry. 2021;82:20m13410.
Chen F, Bertelsen AB, Holm IE, Nyengaard JR, Rosenberg R, Dorph-Petersen KA. Hippocampal volume and cell number in depression, schizophrenia, and suicide subjects. Brain Res. 2020;1727:146546.
Fouquet M, Besson FL, Gonneaud J, La Joie R, Chételat G. Imaging brain effects of APOE4 in cognitively normal individuals across the lifespan. Neuropsychol Rev. 2014;24:290–9.
Jack CR, Petersen RC, Xu YC, O'Brien PC, Waring SC, Tangalos EG, et al. Hippocampal atrophy and apolipoprotein E genotype are independently associated with Alzheimer’s disease. Ann Neurol. 1998;43:303–10.
Operto G, Chupin M, Batrancourt B, Habert MO, Colliot O, Benali H, et al. CATI: a large distributed infrastructure for the neuroimaging of cohorts. Neuroinformatics. 2016;14:253–64.
Pilipenko V, Narbute K, Pupure J, Rumaks J, Jansone B, Klusa V. Neuroprotective action of diazepam at very low and moderate doses in Alzheimer’s disease model rats. Neuropharmacology. 2019;144:319–26.
Arbo BD, Marques CV, Ruiz-Palmero I, Ortiz-Rodriguez A, Ghorbanpoor S, Arevalo MA, et al. 4′-Chlorodiazepam is neuroprotective against amyloid-beta through the modulation of survivin and bax protein expression in vitro. Brain Res. 2016;1632:91–97.
Haberman RP, Branch A, Gallagher M. Targeting neural hyperactivity as a treatment to stem progression of late-onset Alzheimer’s disease. Neurotherapeutics. 2017;14:662–76.
Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, et al. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat Neurosci. 2011;14:750–6.
Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, et al. Endocytosis is required for synaptic activity-dependent release of amyloid-β in vivo. Neuron. 2008;58:42–51.
Busche MA, Konnerth A. Impairments of neural circuit function in Alzheimer’s disease. Philos Trans R Soc B Biol Sci. 2016;371:20150429.
Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, et al. Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron. 2005;48:913–22.
Yuan P, Grutzendler J. Attenuation of -amyloid deposition and neurotoxicity by chemogenetic modulation of neural activity. J Neurosci. 2016;36:632–41.
Veenman L, Papadopoulos V, Gavish M. Channel-Like Functions of the 18-kDa Translocator Protein (TSPO): regulation of apoptosis and steroidogenesis as part of the host-defense response. Curr Pharm Des. 2007;13:2385–405.
Arbo BD, Benetti F, Garcia-Segura LM, Ribeiro MF. Therapeutic actions of translocator protein (18 kDa) ligands in experimental models of psychiatric disorders and neurodegenerative diseases. J. Steroid Biochem Mol Biol. 2015;154:68–74.
Huhtaniska S, Korkala I, Heikka T, Björnholm L, Lehtiniemi H, Hulkko AP, et al. Antipsychotic and benzodiazepine use and brain morphology in schizophrenia and affective psychoses – Systematic reviews and birth cohort study. Psychiatry Res Neuroimaging. 2018;281:43–52.
Licata SC, Shinday NM, Huizenga MN, Darnell SB, Sangrey GR, Rudolph U, et al. Alterations in brain-derived neurotrophic factor in the mouse hippocampus following acute but not repeated benzodiazepine treatment. PLoS ONE. 2013;8:e84806.
Jack CR Jr, Wiste HJ, Knopman DS, Vemuri P, Mielke MM, Weigand SD, et al. Rates of -amyloid accumulation are independent of hippocampal neurodegeneration. Neurology. 2014;82:1605–12.
Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. GABAA receptor subunits in the rat hippocampus I: Immunocytochemical distribution of 13 subunits. Neuroscience. 1997;80:987–1000.
Kreutzmann JC, Havekes R, Abel T, Meerlo P. Sleep deprivation and hippocampal vulnerability: changes in neuronal plasticity, neurogenesis and cognitive function. Neuroscience. 2015;309:173–90.
Acknowledgements
The MEMENTO cohort is sponsored by Bordeaux University Hospital (coordination: CIC1401-EC, Bordeaux) and was funded through research grants from the Fondation Plan Alzheimer (Alzheimer Plan 2008–2012), the French ministry of research,higher education and innovation (Plan Maladies Neurodégénératives (2016–2019)). The MEMENTO cohort has received funding support from AVID, GE Healthcare, and FUJIREBIO through private-public partnerships. The Insight-PreAD sub-study was promoted by INSERM in collaboration with the Institut du Cerveau et de la Moelle épinière, Institut Hospitalo-Universitaire, and Pfizer and has received support within the “Investissement d’Avenir” (ANR-10-AIHU-06) program. Sponsor and funders were not involved in the study conduct, analysis and interpretation of data. This work was undertaken using resources on the Dementias Platform UK (DPUK) Data Portal; the Medical Research Council supports DPUK through grant MR/L023784/2.” The study was supported in part by the French National Agency for Research (“Investissements d’Avenir” no. ANR-11-LABX-0018-01), IRON.
Author information
Authors and Affiliations
Consortia
Contributions
QG, VB, and TD gathered data, which were analyzed and interpreted by QG, TD, and VB. ML and MC performed neuroimaging post-treatment and analyses. MOH, ACB, AS, CB, NA, MJR, LB, FA, JPC, VG, have participated substantial contributions to the conception, QG and TD wrote the article. All authors (QG, VB, ML, MOH, MC, JD, TL, GR, RD, SB, ACB, AS, CB, NA, MJR, LB, FA, JPC, VG, WE, VC, BG, TD) participated in drafting, editing, and revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
TD reports personal fees from Lundbeck, Otsuka and Eisai. GR reports personal fees from Janssen & Janssen and Ostuka. RD reports personal fees from Janssen & Janssen, lundbeck, Lilly, BMS, Servier, Eisai and Biogen. WE reports personal fees from Eisai, Janssen, Lundbeck, Otsuka, UCB, Roche and Chugai. VC reports personal fees from Janssen and Bristol Meyers Squibb. All other authors declare no competing interests. As far as we are aware of, among the pharmaceutical companies mentioned here, only Roche is involved in the production of benzodiazepines (namely diazepam, in its marketed form of Valium in France). Neither Roche nor any of the other pharmaceutical companies mentioned here were consulted regarding the planning or analysis of the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Gallet, Q., Bouteloup, V., Locatelli, M. et al. Benzodiazepine use and neuroimaging markers of Alzheimer’s disease in nondemented older individuals: an MRI and 18F Florbetapir PET study in the MEMENTO cohort. Neuropsychopharmacol. 47, 1114–1120 (2022). https://doi.org/10.1038/s41386-021-01246-5
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41386-021-01246-5
This article is cited by
-
Psychoactive substances: novel molecular insights and therapeutic potential for Alzheimer's disease
Translational Neurodegeneration (2025)
-
Benzodiazepine use in relation to long-term dementia risk and imaging markers of neurodegeneration: a population-based study
BMC Medicine (2024)
-
Benzodiazepines in Alzheimer’s disease: beneficial or detrimental effects
Inflammopharmacology (2023)
-
Large multi-ethnic genetic analyses of amyloid imaging identify new genes for Alzheimer disease
Acta Neuropathologica Communications (2023)