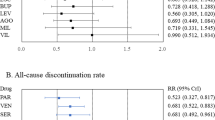
Abstract
This study was a 10-week double-blind, placebo-controlled pilot trial of mirtazapine for anxiety in youth with autism spectrum disorder (ASD). Participants were ages 5 to 17 years with ASD and clinically significant anxiety (Pediatric Anxiety Rating Scale [PARS] score ≥10). Thirty participants were randomized to mirtazapine (7.5–45 mg/day) or placebo in a 2:1 ratio. The co-primary outcome measures were the PARS and the Clinical Global Impressions-Improvement subscale (CGI-I). Mirtazapine resulted in a statistically significant within group decrease in anxiety on the PARS (ES 1.76, p < 0.001). The improvement in PARS score for mirtazapine versus placebo was clinically meaningful but not statistically significant (ES = 0.63, p = 0.64). Forty-seven percent of participants assigned to mirtazapine (95% CI 22%: 74%) and 20% assigned to placebo (95% CI 2%: 60%) were rated “much improved” (CGI-I = 2) or “very much improved” (CGI-I = 1) for anxiety, p = 0.46. No statistically significant differences in mean 10-week changes between mirtazapine and placebo occurred on any outcome measure. There were no statistically significant differences in adverse effect frequency between mirtazapine and placebo. The results are consistent with mirtazapine’s safety and tolerability and meet three of four pre-specified indicators of efficacy (statistically significant change in total PARS score for mirtazapine, numerically greater reduction in total PARS score for mirtazapine than placebo, numerically higher number of responders to mirtazapine than placebo, but not greater than 50% of participants receiving mirtazapine rated as responders). Implementation of a larger randomized controlled trial of mirtazapine for the treatment of anxiety in this population is supported.
Clinical trial registration information: Mirtazapine Treatment of Anxiety in Children and Adolescents with Pervasive Developmental Disorders; https://clinicaltrials.gov; NCT01302964.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
American Psychiatric Association: Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
Kerns CM, Winder-Patel B, Iosif AM, Nordahl CW, Heath B, Solomon M, et al. Clinically significant anxiety in children with autism spectrum disorder and varied intellectual functioning. J Clin Child Adolesc Psychol. 2020. https://doi.org/10.1080/15374416.2019.1703712.
Kirsch AC, Huebner ARS, Mehta SQ, Howie FR, Weaver AL, Myers SM, et al. Association of comorbid mood and anxiety disorders with autism spectrum disorder. JAMA Pediatr. 2020;174:63–70.
van Steensel FJA, Bögels SM, Perrin S. Anxiety disorders in children and adolescents with autistic spectrum disorders: a meta-analysis. Clin Child Fam Psychol Rev. 2011;14:302–17.
van Steensel FJA, Bögels SM, Dirksen CD. Anxiety and quality of life: clinically anxious children with and without autism spectrum disorders compared. J Clin Child Adolesc Psychol. 2012;41:731–8.
Delli CKS, Polychronopoulou SA, Kolaitis GA, Antoniou, SG A-. Review of interventions for the management of anxiety symptoms in children with ASD. Neurosci Biobehav Rev. 2018;95:449–63.
Steingard RJ, Zimnitzky B, DeMaso DR, Bauman ML, Bucci JP. Sertraline treatment of transition-associated anxiety and agitation in children with autistic disorder. J Child Adolesc Psychopharmacol. 1997;7:9–15.
Couturier JL, Nicolson R. A retrospective assessment of citalopram in children and adolescents with pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2002;12:243–8.
Martin A, Koenig K, Anderson GM, Scahill L. Low-dose fluvoxamine treatment of children and adolescents with pervasive developmental disorders: a prospective, open-label study. J Autism Dev Disord. 2003;33:77–85.
Herscu P, Handen BL, Arnold LE, Snape MF, Bregman JD, Ginsberg L, et al. The SOFIA Study: Negative multi-center study of low dose fluoxetine on repetitive behaviors in children and adolescents with autistic disorder. J Autism Dev Disord. 2020;50:3233–44.
Ceranoglu TA, Wozniak J, Fried R, Galdo M, Hoskova B, Fong MD, et al. A retrospective chart review of buspirone for the treatment of anxiety in psychiatrically referred youth with high-functioning autism spectrum disorder. J Child Adolesc Psychopharmacol. 2019;29:28–33.
Buitelaar JK, van der Gaag RJ, van der Hoeven J. Buspirone in the management of anxiety and irritability in children with pervasive developmental disorders: results of an open-label study. J Clin Psychiatry. 1998;59:56–59.
Chugani DC, Chugani HT, Wiznitzer M, Parikh S, Evans PA, Hansen RL, et al. Efficacy of low-dose buspirone for restricted and repetitive behavior in young children with autism spectrum disorder: A randomized trial. J Pediatr. 2016;170:45–53.e1-4.
Schatzberg A, DeBattista C, Manual of clinical psychopharmacology. 8th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2015.
de Boer T. The pharmacologic profile of mirtazapine. J Clin Psychiatry. 1996;57:19–25.
Gambi F, De Berardis D, Campanella D, Carano A, Sepede G, Salini G, et al. Mirtazapine treatment of generalized anxiety disorder: A fixed dose, open label study. J Psychopharmacol. 2005;19:483–7.
Mrakotsky C, Masek B, Biederman J, Raches D, Hsin O, Forbes P, et al. Prospective open-label pilot trial of mirtazapine in children and adolescents with social phobia. J Anxiety Disord. 2008;22:88–97.
Thompson C. Mirtazapine versus selective serotonin reuptake inhibitors. J Clin Psychiatry 1999;60:18–22. Suppl 17discussion 46-8
Owen J, Nemeroff C. New antidepressants and the cytochrome P450 system: focus on venlafaxine, nefazodone, and mirtazapine. Depress Anxiety. 1998;7:24–32.
Posey DJ, Guenin KD, Kohn AE, Swiezy NB, McDougle CJ. A naturalistic open-label study of mirtazapine in autistic and other pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2001;11:267–77.
Janzen HL, Obrzut JE, Marusiak CW. Test Review: Roid, G. H. (2003). Stanford-Binet Intelligence Scales, Fifth Edition (SB:V). Itasca, IL: Riverside Publishing. Can J Sch Psychol. 2004;19:235–44.
Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–85.
Anxiety Disorders Interview Schedule (ADIS-IV) Child/Parent Version Combination Specimen Set - Wendy K. Silverman; Anne Marie Albano - Oxford University Press. https://global.oup.com/academic/product/anxiety-disorders-interview-schedule-adis-iv-childparent-version-combination-specimen-set-9780195186741?cc=us&lang=en&. Accessed 23 June 2020.
PPVT-4 Peabody Picture Vocabulary Test 4th Edition. https://www.pearsonassessments.com/store/usassessments/en/Store/Professional-Assessments/Academic-Learning/Brief/Peabody-Picture-Vocabulary-Test-%7C-Fourth-Edition/p/100000501.html. Accessed 23 June 2020.
Vineland Adaptive Behavior Scales – Second Edition (VinelandTM-II) - TSLAT. http://www.txautism.net/evaluations/vineland-adaptive-behavior-scales-second-edition-vinelandtm-ii. Accessed 23 June 2020.
CGI - Clinical Global Impressions scale. https://eprovide.mapi-trust.org/instruments/clinical-global-impressions-scale. Accessed 23 June 2020.
Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M. Psychometric properties of the screen for child anxiety related emotional disorders (SCARED): A replication study. J Am Acad Child Adolesc Psychiatry. 1999;38:1230–6.
Sukhodolsky DG, Scahill L, Gadow KD, Arnold LE, Aman MG, McDougle CJ, et al. Parent-rated anxiety symptoms in children with pervasive developmental disorders: Frequency and association with core autism symptoms and cognitive functioning. J Abnorm Child Psychol. 2008;36:117–28.
Aman MG, Singh NN, Stewart AW, Field CJ. The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. Am J Ment Defic. 1985;89:485–91.
Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep 2000;23:1043–51.
Wagner A, Lecavalier L, Arnold LE, Aman MG, Scahill L, Stigler KA, et al. Developmental disabilities modification of the children’s global assessment scale. Biol Psychiatry. 2007;61:504–11.
Borm GF, Fransen J, Lemmens WAJG A simple sample size formula for analysis of covariance in randomized clinical trials. https://doi.org/10.1016/j.jclinepi.2007.02.006.
Pine DS, Walkup JT, Labellarte MJ, Riddle MA, Greenhill L, Klein R, et al. Fluvoxamine for the treatment of anxiety disorders in children and adolescents. N. Engl J Med. 2001;344:1279–85.
Hidalgo RB, Tupler LA, Davidson JRT. An effect-size analysis of pharmacologic treatments for generalized anxiety disorder. J Psychopharmacol. 2007;21:864–72.
Funding
This work was funded by Autism Speaks.
Author information
Authors and Affiliations
Contributions
Christopher J. McDougle. Substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work. Drafting the work or revising it critically for important intellectual content. Final approval of the version to be published. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Robyn P. Thom. Substantial contributions to the conception or design of the work; or the acquisition, analysis or interpretation of data for the work. Drafting the work or revising it critically for important intellectual content. Final approval of the version to be published. Caitlin T. Ravichandran. Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. Drafting the work or revising it critically for important intellectual content. Final approval of the version to be published. Michelle L. Palumbo. Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. Final approval of the version to be published. Laura C. Politte. Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. Final approval of the version to be published. Jennifer E. Mullett. Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. Final approval of the version to be published. Christopher J. Keary. Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. Final approval of the version to be published. Craig A. Erickson. Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. Final approval of the version to be published. Kimberly A. Stigler. Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. Final approval of the version to be published. Lauren Mathieu-Frasier. Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. Final approval of the version to be published. David J. Posey. Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work. Drafting of the work or revising it critically for important intellectual content. Final approval of the version to be published. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
Dr. McDougle is a consultant to Precidiag, Receptor Life Sciences, and Sage Therapeutics, and receives royalties from Oxford University Press and Springer Publishing. Dr. Thom receives research support from Precidiag. Dr. Palumbo receives research support from Otsuka Pharmaceuticals. Dr. Keary serves on the advisory board and receives research support from Ovid Therapeutics. Dr. Erickson is a consultant to Autifony, Stalicla, Scitoto Bioscience, Impel, and Confluence Pharmaceuticals. He is an inventor on patents describing methods of treatment in neurodevelopmental disorders held by the Cincinnati Children’s Hospital Research Foundation. Drs. Ravichandran and Politte, Ms. Mullett, Dr. Stigler, Ms. Mathieu-Frasier, and Dr. Posey have no disclosures or conflicts of interest to report.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
McDougle, C.J., Thom, R.P., Ravichandran, C.T. et al. A randomized double-blind, placebo-controlled pilot trial of mirtazapine for anxiety in children and adolescents with autism spectrum disorder. Neuropsychopharmacol. 47, 1263–1270 (2022). https://doi.org/10.1038/s41386-022-01295-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-022-01295-4
This article is cited by
-
Pharmacological treatment in autism: a proposal for guidelines on common co-occurring psychiatric symptoms
BMC Medicine (2025)
-
Patterns in Medication Use for Treatment of Depression in Autistic Spectrum Disorder
Journal of Autism and Developmental Disorders (2025)
-
Randomized controlled trial of propranolol on social communication and anxiety in children and young adults with autism spectrum disorder
Psychopharmacology (2024)
-
Signalling pathways in autism spectrum disorder: mechanisms and therapeutic implications
Signal Transduction and Targeted Therapy (2022)