Abstract
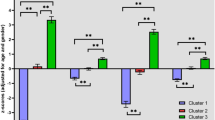
Some patients with schizophrenia have severe cognitive impairment and functional deficits that require long-term institutional care. The patterns of brain-behavior alterations in these individuals, and their differences from patients living successfully in the community, remain poorly understood. Previous cognition-based studies for stratifying schizophrenia patients highlight the importance of subcortical structures in the context of illness heterogeneity. In the present study, subcortical volumes from 96 institutionalized patients with long-term schizophrenia were evaluated using cluster analysis to test for heterogeneity. These data were compared to those from two groups of community-dwelling individuals with schizophrenia for comparison purposes, including 68 long-term ill and 126 first-episode individuals. A total of 290 demographically matched healthy participants were included as normative references at a 1:1 ratio for each patient sample. A subtype of institutionalized patients was identified based on their pattern of subcortical alterations. Using a machine learning algorithm developed to discriminate the two groups of institutionalized patients, all three patient samples were found to have similar rates of patients assigned to the two subtypes (approximately 50% each). In institutionalized patients, only the subtype with the identified pattern of subcortical alterations had greater neocortical and cognitive abnormalities than those in the similarity classified community-dwelling patients with long-term illness. Thus, for the subtype of patients with a distinctive pattern of subcortical alterations, when the distinct pattern of subcortical alterations is present and particularly severe, it is associated with cognitive impairments that may contribute to persistent disability and institutionalization.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Wakuda T, Takei N. ‘Opening doors’ for long-term institutionalised patients with schizophrenia in Japan. Acta Psychiatr Scand. 2021;143:277–78.
Nemoto T, Niimura H, Ryu Y, Sakuma K, Mizuno M. Long-term course of cognitive function in chronically hospitalized patients with schizophrenia transitioning to community-based living. Schizophr Res. 2014;155:90–5.
Kubota M, van Haren NE, Haijma SV, Schnack HG, Cahn W, Hulshoff Pol HE, et al. Association of IQ changes and progressive brain changes in patients with schizophrenia. JAMA Psychiatry. 2015;72:803–12.
Nestor PG, Kubicki M, Nakamura M, Niznikiewicz M, Levitt JJ, Shenton ME, et al. Neuropsychological variability, symptoms, and brain imaging in chronic schizophrenia. Brain Imaging Behav. 2013;7:68–76.
Antonova E, Kumari V, Morris R, Halari R, Anilkumar A, Mehrotra R, et al. The relationship of structural alterations to cognitive deficits in schizophrenia: a voxel-based morphometry study. Biol Psychiatry. 2005;58:457–67.
Halverson TF, Orleans-Pobee M, Merritt C, Sheeran P, Fett AK, Penn DL. Pathways to functional outcomes in schizophrenia spectrum disorders: Meta-analysis of social cognitive and neurocognitive predictors. Neurosci Biobehav Rev. 2019;105:212–19.
Wojtalik JA, Smith MJ, Keshavan MS, Eack SM. A systematic and meta-analytic review of neural correlates of functional outcome in schizophrenia. Schizophr Bull. 2017;43:1329–47.
Xie T, Zhang X, Tang X, Zhang H, Yu M, Gong G, et al. Mapping convergent and divergent cortical thinning patterns in patients with deficit and nondeficit schizophrenia. Schizophr Bull. 2019;45:211–21.
Green MJ, Girshkin L, Kremerskothen K, Watkeys O, Quide Y. A Systematic Review of Studies Reporting Data-Driven Cognitive Subtypes across the Psychosis Spectrum. Neuropsychol Rev. 2020;30:446–60.
Bowen EFW, Burgess JL, Granger R, Kleinman JE, Rhodes CH. DLPFC transcriptome defines two molecular subtypes of schizophrenia. Transl Psychiatry. 2019;9:147.
Schwarz E, van Beveren NJ, Ramsey J, Leweke FM, Rothermundt M, Bogerts B, et al. Identification of subgroups of schizophrenia patients with changes in either immune or growth factor and hormonal pathways. Schizophr Bull. 2014;40:787–95.
Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, et al. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 2016;173:373–84.
Chand GB, Dwyer DB, Erus G, Sotiras A, Varol E, Srinivasan D, et al. Two distinct neuroanatomical subtypes of schizophrenia revealed using machine learning. Brain. 2020;143:1027–38.
Xiao Y, Liao W, Long Z, Tao B, Zhao Q, Luo C, et al. Subtyping schizophrenia patients based on patterns of structural brain alterations. Schizophr Bull. 2022;48:241–50.
Takahashi T, Tsugawa S, Nakajima S, Plitman E, Chakravarty MM, Masuda F, et al. Thalamic and striato-pallidal volumes in schizophrenia patients and individuals at risk for psychosis: A multi-atlas segmentation study. Schizophr Res. 2020. https://doi.org/10.1016/j.schres.2020.04.016
Koshiyama D, Fukunaga M, Okada N, Yamashita F, Yamamori H, Yasuda Y, et al. Role of subcortical structures on cognitive and social function in schizophrenia. Sci Rep. 2018;8:1183.
Koshiyama D, Fukunaga M, Okada N, Yamashita F, Yamamori H, Yasuda Y, et al. Subcortical association with memory performance in schizophrenia: a structural magnetic resonance imaging study. Transl Psychiatry. 2018;8:20.
Weinberg D, Lenroot R, Jacomb I, Allen K, Bruggemann J, Wells R, et al. Cognitive subtypes of schizophrenia characterized by differential brain volumetric reductions and cognitive decline. JAMA Psychiatry. 2016;73:1251–59.
Van Rheenen TE, Cropley V, Zalesky A, Bousman C, Wells R, Bruggemann J, et al. Widespread volumetric reductions in schizophrenia and schizoaffective patients displaying compromised cognitive abilities. Schizophr Bull. 2018;44:560–74.
Beste C, Moll CKE, Potter-Nerger M, Munchau A. Striatal microstructure and its relevance for cognitive control. Trends Cogn Sci. 2018;22:747–51.
Hwang K, Bertolero MA, Liu WB, D’Esposito M. The human thalamus is an integrative hub for functional brain networks. J Neurosci. 2017;37:5594–607.
Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–41.
Hill SK, Reilly JL, Keefe RS, Gold JM, Bishop JR, Gershon ES, et al. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am J Psychiatry. 2013;170:1275–84.
Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, et al. Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013;170:1263–74.
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76.
Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The brief assessment of cognition in schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68:283–97.
Keefe RS, Harvey PD, Goldberg TE, Gold JM, Walker TM, Kennel C, et al. Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS). Schizophr Res. 2008;102:108–15.
Wang LJ, Lin PY, Lee Y, Huang YC, Hsu ST, Hung CF, et al. Validation of the Chinese version of brief assessment of cognition in schizophrenia. Neuropsychiatr Dis Treat. 2016;12:2819–26.
Fischl B. FreeSurfer. Neuroimage. 2012;62:774–81.
Rosen AFG, Roalf DR, Ruparel K, Blake J, Seelaus K, Villa LP, et al. Quantitative assessment of structural image quality. Neuroimage. 2018;169:407–18.
Arthur D, Vassilvitskii S. k-means++: the advantages of careful seeding. Society for Industrial and Applied Mathematics: New Orleans, Louisiana; 2007.
Rousseeuw PJ. Silhouettes - a graphical aid to the interpretation and validation of cluster-analysis. J Computational Appl Math. 1987;20:53–65.
Breiman L. Random forests. Mach Learn. 2001;45:5–32.
R Core Team. R: A Language and Environment for Statistical Computing. 4.0.2 ed. R Foundation for Statistical Computing; 2020.
Hudgens-Haney ME, Clementz BA, Ivleva EI, Keshavan MS, Pearlson GD, Gershon ES, et al. Cognitive impairment and diminished neural responses constitute a biomarker signature of negative symptoms in psychosis. Schizophr Bull. 2020;46:1269–81.
Ivleva EI, Clementz BA, Dutcher AM, Arnold SJM, Jeon-Slaughter H, Aslan S, et al. Brain structure biomarkers in the psychosis biotypes: findings from the bipolar-schizophrenia network for intermediate phenotypes. Biol Psychiatry. 2017;82:26–39.
Harvey PD, Silverman JM, Mohs RC, Parrella M, White L, Powchik P, et al. Cognitive decline in late-life schizophrenia: a longitudinal study of geriatric chronically hospitalized patients. Biol Psychiatry. 1999;45:32–40.
Kurtz MM. Neurocognitive impairment across the lifespan in schizophrenia: an update. Schizophr Res. 2005;74:15–26.
Harvey PD, Reichenberg A, Bowie CR, Patterson TL, Heaton RK. The course of neuropsychological performance and functional capacity in older patients with schizophrenia: influences of previous history of long-term institutional stay. Biol Psychiatry. 2010;67:933–9.
Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168:472–85.
Kambeitz-Ilankovic L, Betz LT, Dominke C, Haas SS, Subramaniam K, Fisher M, et al. Multi-outcome meta-analysis (MOMA) of cognitive remediation in schizophrenia: Revisiting the relevance of human coaching and elucidating interplay between multiple outcomes. Neurosci Biobehav Rev. 2019;107:828–45.
Hill SK, Schuepbach D, Herbener ES, Keshavan MS, Sweeney JA. Pretreatment and longitudinal studies of neuropsychological deficits in antipsychotic-naïve patients with schizophrenia. Schizophr Res. 2004;68:49–63.
Islam MA, Habtewold TD, van Es FD, Quee PJ, van den Heuvel ER, Alizadeh BZ, et al. Long-term cognitive trajectories and heterogeneity in patients with schizophrenia and their unaffected siblings. Acta Psychiatr Scand. 2018;138:591–604.
Zhang W, Deng W, Yao L, Xiao Y, Li F, Liu J, et al. Brain structural abnormalities in a group of never-medicated patients with long-term schizophrenia. Am J Psychiatry. 2015;172:995–1003.
Cetin-Karayumak S, Di Biase MA, Chunga N, Reid B, Somes N, Lyall AE, et al. White matter abnormalities across the lifespan of schizophrenia: a harmonized multi-site diffusion MRI study. Mol Psychiatry. 2020;25:3208–19.
Fett AJ, Velthorst E, Reichenberg A, Ruggero CJ, Callahan JL, Fochtmann LJ, et al. Long-term changes in cognitive functioning in individuals with psychotic disorders: findings from the suffolk county mental health project. JAMA Psychiatry. 2019;77:387–96.
Pergola G, Danet L, Pitel AL, Carlesimo GA, Segobin S, Pariente J, et al. The regulatory role of the human mediodorsal thalamus. Trends Cogn Sci. 2018;22:1011–25.
Brown LL, Schneider JS, Lidsky TI. Sensory and cognitive functions of the basal ganglia. Curr Opin Neurobiol. 1997;7:157–63.
Liu C, Cao B, Yu R, Sim K. Basal ganglia volumetric changes in psychotic spectrum disorders. J Affect Disord. 2019;255:150–57.
Jorgensen KN, Nesvag R, Gunleiksrud S, Raballo A, Jonsson EG, Agartz I. First- and second-generation antipsychotic drug treatment and subcortical brain morphology in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2016;266:451–60.
Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC. Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry. 1998;155:1711–7.
Elkashef AM, Buchanan RW, Gellad F, Munson RC, Breier A. Basal ganglia pathology in schizophrenia and tardive dyskinesia: an MRI quantitative study. Am J Psychiatry. 1994;151:752–5.
van Erp TG, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21:547–53.
Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39:1129–38.
Chua SE, Deng Y, Chen EY, Law CW, Chiu CP, Cheung C, et al. Early striatal hypertrophy in first-episode psychosis within 3 weeks of initiating antipsychotic drug treatment. Psychol Med. 2009;39:793–800.
Ebdrup BH, Glenthoj B, Rasmussen H, Aggernaes B, Langkilde AR, Paulson OB, et al. Hippocampal and caudate volume reductions in antipsychotic-naive first-episode schizophrenia. J Psychiatry Neurosci. 2010;35:95–104.
Chopra S, Fornito A, Francey SM, O'Donoghue B, Cropley V, Nelson B, et al. Differentiating the effect of antipsychotic medication and illness on brain volume reductions in first-episode psychosis: A Longitudinal, Randomised, Triple-blind, Placebo-controlled MRI Study. Neuropsychopharmacology. 2021;46:1494–501.
Kim J, Plitman E, Iwata Y, Nakajima S, Mar W, Patel R, et al. Neuroanatomical profiles of treatment-resistance in patients with schizophrenia spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2020;99:109839.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 82120108014 [to SL], 82071908 [to SL], 81761128023 [to QG], 81621003 [to QG], and 81901705 [to YX]), Chinese Academy of Medical Sciences (Project No. 2021-12M-C&T-A-022 [to SL]), Sichuan Science and Technology Program (Project No. 2021JDTD0002 [to SL]), and 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Project Nos. ZYYC08001 [to SL] and ZYJC18020 [to SL]). This work was supported by the National Institute of Mental Health (NIMH) through its support of the Bipolar–Schizophrenia Network for Intermediate Phenotypes (Grant Nos. MH077851 [to CAT], MH078113 [to MSK], MH077945 [to GDP], MH096942 [to BAC], MH077862 [to JAS] and MH096957 [to ESG]). MSK is supported by research grants from the NIMH and the Bear and Natalia Foundations. EII is supported by a grant from the NIMH (Grant No. 1K23 MH102656). JAS is supported by the University of Cincinnati Schizophrenia Research Fund. SL, JAS, and YX acknowledge the support from Alexander von Humboldt Foundation, and SL acknowledges the support from Chang Jiang Scholars (Program No. T2019069).
Author information
Authors and Affiliations
Contributions
Conception: QZ, SL, QG, and JAS; Methodological development and statistical analysis: QZ, HC, WZ, and JAS; Data collection, acquisition, and interpretation: all authors; Manuscript draft: QZ, SL, JAS, and HC; Critical revisions of the manuscript and final approval of this version to be published: all authors
Corresponding authors
Ethics declarations
Competing interests
WZ, SYL, and JAS consulted to VeraSci. CAT has served on the advisory board for drug development for Intra-Cellular Therapies, Inc., as an ad hoc consultant for Eli Lilly, Sunovion, Astellas, Pfizer, and Merck, has been a council member and unpaid volunteer for the National Alliance on Mental Illness, and served as deputy editor for the American Psychiatric Association. MSK has received research support from Sunovion and GlaxoSmithKline. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhao, Q., Cao, H., Zhang, W. et al. A subtype of institutionalized patients with schizophrenia characterized by pronounced subcortical and cognitive deficits. Neuropsychopharmacol. 47, 2024–2032 (2022). https://doi.org/10.1038/s41386-022-01300-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-022-01300-w
This article is cited by
-
Two structural alteration patterns of the thalamus in patients with schizophrenia
BMC Medicine (2025)
-
Morphological alterations of the thymus gland in individuals with schizophrenia
Molecular Psychiatry (2025)
-
Subtyping schizophrenia via machine learning by using structural neuroimaging
Translational Psychiatry (2025)
-
Distinct neuroimaging subtypes of ADHD among adolescents based on semi-supervised learning
Translational Psychiatry (2025)
-
Multivariate associations between neuroanatomy and cognition in unmedicated and medicated individuals with schizophrenia
Schizophrenia (2024)