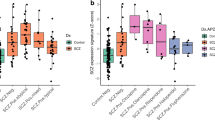
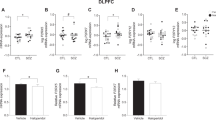
Abstract
Antipsychotic drugs (APDs) are effective in treating positive symptoms of schizophrenia (SCZ). However, they have a substantial impact on postmortem studies. As most cohorts lack samples from drug-naive patients, many studies, rather than understanding SCZ pathophysiology, are analyzing the drug effects. We hypothesized that comparing SCZ-altered and APD-influenced signatures derived from the same cohort can provide better insight into SCZ pathophysiology. For this, we performed LCMS-based proteomics on dorsolateral prefrontal cortex (DLPFC) samples from control and SCZ subjects and used statistical approaches to identify SCZ-altered and APD-influenced proteomes, validated experimentally using independent cohorts and published datasets. Functional analysis of both proteomes was contrasted at the biological-pathway, cell-type, subcellular-synaptic, and drug-target levels. In silico validation revealed that the SCZ-altered proteome was conserved across several studies from the DLPFC and other brain areas. At the pathway level, SCZ influenced changes in homeostasis, signal-transduction, cytoskeleton, and dendrites, whereas APD influenced changes in synaptic-signaling, neurotransmitter-regulation, and immune-system processes. At the cell-type level, the SCZ-altered and APD-influenced proteomes were associated with two distinct striatum-projecting layer-5 pyramidal neurons regulating dopaminergic-secretion. At the subcellular synaptic level, compensatory pre- and postsynaptic events were observed. At the drug-target level, dopaminergic processes influenced the SCZ-altered upregulated-proteome, whereas nondopaminergic and a diverse array of non-neuromodulatory mechanisms influenced the downregulated-proteome. Previous findings were not independent of the APD effect and thus require re-evaluation. We identified a hyperdopaminergic cortex and drugs targeting the cognitive SCZ-symptoms and discussed their influence on SCZ pathology in the context of the cortico-striatal pathway.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data and materials availability
All analyzed data are available in the main text or the supplementary materials. The raw data are available from the corresponding author upon reasonable request.
References
Wu EQ, Birnbaum HG, Shi L, Ball DE, Kessler RC, Moulis M, et al. The economic burden of schizophrenia in the United States in 2002. J Clin Psychiatry. 2005;66:1122–9.
Kolluri N, Sun Z, Sampson AR, Lewis DA. Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2005;162:1200–2.
Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12:386–94.
Laruelle M, Kegeles LS, Abi-Dargham A. Glutamate, dopamine, and schizophrenia. Ann NY Acad Sci. 2003;1003:138–58.
Carboni L, Domenici E. Proteome effects of antipsychotic drugs: Learning from preclinical models. Proteom Clin Appl. 2016;10:430–41.
Enwright JF III, Lewis DA. Similarities in Cortical Transcriptome Alterations Between Schizophrenia and Bipolar Disorder Are Related to the Presence of Psychosis. Schizophrenia Bull. 2021;47:1442–51.
Cosi C, Waget A, Rollet K, Tesori V, Newman-Tancredi A. Clozapine, ziprasidone and aripiprazole but not haloperidol protect against kainic acid-induced lesion of the striatum in mice, in vivo: Role of 5-HT1A receptor activation. Brain Res. 2005;1043:32–41.
Chertkow Y, Weinreb O, Youdim MB, Silver H. The effect of chronic co-administration of fluvoxamine and haloperidol compared to clozapine on the GABA system in the rat frontal cortex. Int J Neuropsychopharmacol. 2006;9:287–96.
Davis AP, Grondin CJ, Johnson RJ, Sciaky D, Wiegers J, Wiegers TC, et al. Comparative Toxicogenomics Database (CTD): update 2021. Nucleic Acids Res. 2021;49:D1138–D43.
Subramanian A, Narayan R, Corsello SM, Peck DD, Natoli TE, Lu X, et al. A Next Generation Connectivity Map: L1000 Platform and the First 1,000,000 Profiles. Cell 2017;171:1437–52 e17.
Roberts RC, Roche JK, Conley RR, Lahti AC. Dopaminergic synapses in the caudate of subjects with schizophrenia: Relationship to treatment response. Synapse 2009;63:520–30.
Pfisterer U, Petukhov V, Demharter S, Meichsner J, Thompson JJ, Batiuk MY, et al. Identification of epilepsy-associated neuronal subtypes and gene expression underlying epileptogenesis. Nat Commun. 2020;11:5038.
Shukla R, Newton DF, Sumitomo A, Zare H, McCullumsmith R, Lewis DA, et al. Molecular Characterization of Depression Trait and State. bioRxiv. 2020:2020.04.24.058610.
Hoffman GE, Schadt EE. variancePartition: Interpreting drivers of variation in complex gene expression studies. BMC Bioinforma. 2016;17:483.
Steffek AE, McCullumsmith RE, Haroutunian V, Meador-Woodruff JH. Cortical expression of glial fibrillary acidic protein and glutamine synthetase is decreased in schizophrenia. Schizophr Res. 2008;103:71–82.
Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE. Glutamate in schizophrenia: A focused review and meta-analysis of ¹H-MRS studies. Schizophr Bull. 2013;39:120–9.
Bartha R, Williamson PC, Drost DJ, Malla A, Carr TJ, Cortese L, et al. Measurement of glutamate and glutamine in the medial prefrontal cortex of never-treated schizophrenic patients and healthy controls by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1997;54:959–65.
Martins-de-Souza D, Gattaz WF, Schmitt A, Maccarrone G, Hunyadi-Gulyás E, Eberlin MN, et al. Proteomic analysis of dorsolateral prefrontal cortex indicates the involvement of cytoskeleton, oligodendrocyte, energy metabolism and new potential markers in schizophrenia. J Psychiatr Res. 2009;43:978–86.
Behan Á, Byrne C, Dunn MJ, Cagney G, Cotter DR. Proteomic analysis of membrane microdomain-associated proteins in the dorsolateral prefrontal cortex in schizophrenia and bipolar disorder reveals alterations in LAMP, STXBP1 and BASP1 protein expression. Mol Psychiatry. 2009;14:601–13.
Pennington K, Dicker P, Dunn MJ, Cotter DR. Proteomic analysis reveals protein changes within layer 2 of the insular cortex in schizophrenia. Proteomics 2008;8:5097–107.
Schubert KO, Föcking M, Cotter DR. Proteomic pathway analysis of the hippocampus in schizophrenia and bipolar affective disorder implicates 14-3-3 signaling, aryl hydrocarbon receptor signaling, and glucose metabolism: potential roles in GABAergic interneuron pathology. Schizophr Res. 2015;167:64–72.
Nesvaderani M, Matsumoto I, Sivagnanasundaram S. Anterior Hippocampus in Schizophrenia Pathogenesis: Molecular Evidence from a Proteome Study. Aust NZ J Psychiatry. 2009;43:310–22.
Sivagnanasundaram S, Crossett B, Dedova I, Cordwell S, Matsumoto I. Abnormal pathways in the genu of the corpus callosum in schizophrenia pathogenesis: a proteome study. Proteom Clin Appl. 2007;1:1291–305.
Clark D, Dedova I, Cordwell S, Matsumoto I. A proteome analysis of the anterior cingulate cortex gray matter in schizophrenia. Mol Psychiatry. 2006;11:459–70.
Clark D, Dedova I, Cordwell S, Matsumoto I. Altered proteins of the anterior cingulate cortex white matter proteome in schizophrenia. Proteom Clin Appl. 2007;1:157–66.
Duncan GE, Zorn S, Lieberman JA. Mechanisms of typical and atypical antipsychotic drug action in relation to dopamine and NMDA receptor hypofunction hypotheses of schizophrenia. Mol Psychiatry. 1999;4:418–28.
Citrome L. Schizophrenia and valproate. Psychopharmacol Bull. 2003;37:74–88. Suppl 2
Wang H, Azuaje F, Bodenreider O, Dopazo J. Gene expression correlation and gene ontology-based similarity: An assessment of quantitative relationships. Proc IEEE Symp Comput Intell Bioinforma Comput Biol. 2004;2004:25–31.
Rangaraju S, Solis GM, Thompson RC, Gomez-Amaro RL, Kurian L, Encalada SE, et al. Suppression of transcriptional drift extends C. elegans lifespan by postponing the onset of mortality. Elife 2015;4:e08833.
Reddy V, Sherif M, Shukla R. Integrating single-cell transcriptomics and microcircuit computer modeling. Curr Opin Pharmacol. 2021;60:34–39.
Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry. 2006;59:929–39.
Koopmans F, van Nierop P, Andres-Alonso M, Byrnes A, Cijsouw T, Coba MP, et al. SynGO: An evidence-based, expert-curated knowledge base for the synapse. Neuron 2019;103:217–34.e4.
Nagy C, Maitra M, Tanti A, Suderman M, Théroux J-F, Davoli MA, et al. Single-nucleus transcriptomics of the prefrontal cortex in major depressive disorder implicates oligodendrocyte precursor cells and excitatory neurons. Nat Neurosci. 2020;23:771–81.
Baker A, Kalmbach B, Morishima M, Kim J, Juavinett A, Li N, et al. Specialized Subpopulations of deep-layer pyramidal neurons in the neocortex: Bridging cellular properties to functional consequences. J Neurosci. 2018;38:5441–55.
Shukla R, Henkel ND, Alganem K, Hamoud AR, Reigle J, Alnafisah RS, et al. Signature-based approaches for informed drug repurposing: Targeting CNS disorders. Neuropsychopharmacology 2021;46:116–30.
Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: A circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25.
Thune JJ, Uylings HB, Pakkenberg B. No deficit in total number of neurons in the prefrontal cortex in schizophrenics. J Psychiatr Res. 2001;35:15–21.
Lewis DA, Sweet RA. Schizophrenia from a neural circuitry perspective: Advancing toward rational pharmacological therapies. J Clin Invest. 2009;119:706–16.
Berretta S. Extracellular matrix abnormalities in schizophrenia. Neuropharmacology 2012;62:1584–97.
JamesEB, IanMK, AaronWG, AnnaYK, Diana O, Victor V, et al. Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. Am J Psychiatry. 2004;161:742–4.
Staudinger MR, Erk S, Walter H. Dorsolateral prefrontal cortex modulates striatal reward encoding during reappraisal of reward anticipation. Cereb Cortex. 2011;21:2578–88.
Kosillo P, Zhang YF, Threlfell S, Cragg SJ. Cortical control of striatal dopamine transmission via striatal cholinergic interneurons. Cereb Cortex. 2016;26:4160–9.
McCutcheon RA, Abi-Dargham A, Howes OD. Schizophrenia, dopamine and the striatum: From biology to symptoms. Trends Neurosci. 2019;42:205–20.
Ballard IC, Murty VP, Carter RM, MacInnes JJ, Huettel SA, Adcock RA. Dorsolateral prefrontal cortex drives mesolimbic dopaminergic regions to initiate motivated behavior. J Neurosci. 2011;31:10340–6.
Seeman P. Dopamine D2 receptors as treatment targets in schizophrenia. Clin Schizophr Relat Psychoses. 2010;4:56–73.
Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science 1997;275:1593–9.
Erickson SL, Lewis DA. Cortical connections of the lateral mediodorsal thalamus in cynomolgus monkeys. J Comp Neurol. 2004;473:107–27.
Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science 1979;205:929–32.
Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–57.
Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: Insights for cognitive dysfunction. Psychopharmacol (Berl). 2004;174:3–16.
Mayada A, JosephNP, RichardEW, ChristineLE, Carrie M, AllanRS, et al. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry. 1999;156:1580–9.
Barbey AK, Koenigs M, Grafman J. Dorsolateral prefrontal contributions to human working memory. Cortex 2013;49:1195–205.
Han S, Becker B, Duan X, Cui Q, Xin F, Zong X, et al. Distinct striatum pathways connected to salience network predict symptoms improvement and resilient functioning in schizophrenia following risperidone monotherapy. Schizophr Res. 2020;215:89–96.
Cox J, Witten IB. Striatal circuits for reward learning and decision-making. Nat Rev Neurosci. 2019;20:482–94.
Anderson EM, Gomez D, Caccamise A, McPhail D, Hearing M. Chronic unpredictable stress promotes cell-specific plasticity in prefrontal cortex D1 and D2 pyramidal neurons. Neurobiol Stress. 2019;10:100152.
Koseska A, Bastiaens PI. Cell signaling as a cognitive process. EMBO J. 2017;36:568–82.
Schwartz JH. Cognitive kinases. Proc Natl Acad Sci USA. 1993;90:8310–3.
Dash PK, Moore AN, Kobori N, Runyan JD. Molecular activity underlying working memory. Learn Mem. 2007;14:554–63.
Loranger AW. Sex difference in age at onset of schizophrenia. Arch Gen Psychiatry. 1984;41:157–61.
Smail MA, Chandrasena SS, Zhang X, Reddy V, Kelley C, Herman JP, et al. Differential vulnerability of anterior cingulate cortex cell-types to diseases and drugs. bioRxiv. 2021:2021.10.26.465972.
Funding
RSA is supported by a predoctoral fellowship from the government of Saudi Arabia.
Author information
Authors and Affiliations
Contributions
RS and RSA conceptualized the study and wrote the manuscript. JR, MAE, JM, and REM participated with RSA in LCMS data generation and processing. SMO and AJF participated with RSA in western blot analysis. All authors participated in writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alnafisah, R.S., Reigle, J., Eladawi, M.A. et al. Assessing the effects of antipsychotic medications on schizophrenia functional analysis: a postmortem proteome study. Neuropsychopharmacol. 47, 2033–2041 (2022). https://doi.org/10.1038/s41386-022-01310-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-022-01310-8
This article is cited by
-
Post-mortem brain analysis reveals altered monoaminergic system in the dorsolateral prefrontal cortex and hippocampus in chronic schizophrenia
Schizophrenia (2026)
-
Cholinergic system in schizophrenia: A systematic review and meta-analysis
Molecular Psychiatry (2025)
-
Antipsychotic drug use complicates assessment of gene expression changes associated with schizophrenia
Translational Psychiatry (2023)
-
Differential vulnerability of anterior cingulate cortex cell types to diseases and drugs
Molecular Psychiatry (2022)