Abstract
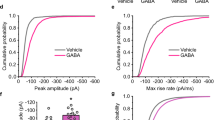
GABA-A receptors (GABAARs) are crucial for development and function of the brain. Altered GABAergic transmission is hypothesized to be involved in neurodevelopmental disorders. Recently, we identified Shisa7 as a GABAAR auxiliary subunit that modulates GABAAR trafficking and GABAergic transmission. However, the underlying molecular mechanisms remain elusive. Here we generated a knock-in (KI) mouse line that is phospho-deficient at a phosphorylation site in Shisa7 (S405) and combined with electrophysiology, imaging and behavioral assays to illustrate the role of this site in GABAergic transmission and plasticity as well as behaviors. We found that expression of phospho-deficient mutants diminished α2-GABAAR trafficking in heterologous cells. Additionally, α1/α2/α5-GABAAR surface expression and GABAergic inhibition were decreased in hippocampal neurons in KI mice. Moreover, chemically induced inhibitory long-term potentiation was abolished in KI mice. Lastly, KI mice exhibited hyperactivity, increased grooming and impaired sleep homeostasis. Collectively, our study reveals a phosphorylation site critical for Shisa7-dependent GABAARs trafficking which contributes to behavioral endophenotypes displayed in neurodevelopmental disorders.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Sieghart W, Savic MM. International union of basic and clinical pharmacology. CVI: GABAA Receptor Subtype- and Function-selective Ligands: Key Issues in Translation to Humans. Pharm Rev. 2018;70:836–78.
Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–8.
Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–29.
Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–43.
Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16:383–406.
Mohler H. GABAA receptors in central nervous system disease: anxiety, epilepsy, and insomnia. J Recept Signal Transduct Res. 2006;26:731–40.
Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia. 2001;42:8–12.
Hines RM, Davies PA, Moss SJ, Maguire J. Functional regulation of GABAA receptors in nervous system pathologies. Curr Opin Neurobiol. 2012;22:552–8.
Maher MP, Matta JA, Gu S, Seierstad M, Bredt DS. Getting a Handle on Neuropharmacology by Targeting Receptor-Associated Proteins. Neuron. 2017;96:989–1001.
Han W, Shepard RD, Lu W. Regulation of GABAARs by Transmembrane Accessory Proteins. Trends Neurosci. 2021;44:152–65.
Castellano D, Shepard RD, Lu W. Looking for Novelty in an “Old” Receptor: Recent Advances Toward Our Understanding of GABAARs and Their Implications in Receptor Pharmacology. Front Neurosci. 2020;14:616298.
Nakamura Y, Morrow DH, Modgil A, Huyghe D, Deeb TZ, Lumb MJ, et al. Proteomic Characterization of Inhibitory Synapses Using a Novel pHluorin-tagged gamma-Aminobutyric Acid Receptor, Type A (GABAA), alpha2 Subunit Knock-in Mouse. J Biol Chem. 2016;291:12394–407.
Farrow P, Khodosevich K, Sapir Y, Schulmann A, Aslam M, Stern-Bach Y, et al. Auxiliary subunits of the CKAMP family differentially modulate AMPA receptor properties. eLife 2015;4:e09693. https://doi.org/10.7554/eLife.09693.
Schmitz LJM, Klaassen RV, Ruiperez-Alonso M, Zamri AE, Stroeder J, Priyanka R-R. et al. The AMPA receptor-associated protein Shisa7 regulates hippocampal synaptic function and contextual memory. eLife. 2017;6:e24192, https://doi.org/10.7554/eLife.24192.
Han W, Li J, Pelkey KA, Pandey S, Chen X, Wang YX, et al. Shisa7 is a GABAA receptor auxiliary subunit controlling benzodiazepine actions. Science. 2019;366:246–50.
Wu K, Han W, Tian Q, Li Y, Lu W. Activity- and sleep-dependent regulation of tonic inhibition by Shisa7. Cell Rep. 2021;34:108899.
Gatto CL, Broadie K Genetic controls balancing excitatory and inhibitory synaptogenesis in neurodevelopmental disorder models. Front Synaptic Neurosci. 2010;2:4.
Lee E, Lee J, Kim E. Excitation/Inhibition imbalance in animal models of autism spectrum disorders. Biol Psychiatry. 2017;81:838–47.
Coghlan S, Horder J, Inkster B, Mendez MA, Murphy DG, Nutt DJ. GABA system dysfunction in autism and related disorders: from synapse to symptoms. Neurosci Biobehav Rev. 2012;36:2044–55.
Kazdoba TM, Leach PT, Yang M, Silverman JL, Solomon M, Crawley JN. Translational mouse models of autism: advancing toward pharmacological therapeutics. Curr Top Behav Neurosci. 2016;28:1–52.
Ahmari SE. Using mice to model Obsessive Compulsive Disorder: From genes to circuits. Neuroscience. 2016;321:121–37.
Tager-Flusberg H, Joseph R, Folstein S. Current directions in research on autism. Ment Retard Dev Disabil Res Rev. 2001;7:21–9.
Buxbaum JD, Silverman JM, Smith CJ, Greenberg DA, Kilifarski M, Reichert J, et al. Association between a GABRB3 polymorphism and autism. Mol Psychiatry. 2002;7:311–6.
Samaco RC, Hogart A, LaSalle JM. Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Hum Mol Genet. 2005;14:483–92.
Fatemi SH, Reutiman TJ, Folsom TD, Thuras PD. GABA(A) receptor downregulation in brains of subjects with autism. J Autism Developmental Disord. 2009;39:223–30.
Fatemi SH, Reutiman TJ, Folsom TD, Rustan OG, Rooney RJ, Thuras PD. Downregulation of GABAA receptor protein subunits alpha6, beta2, delta, epsilon, gamma2, theta, and rho2 in superior frontal cortex of subjects with autism. J Autism Developmental Disord. 2014;44:1833–45.
Mesbah-Oskui L, Penna A, Orser BA, Horner RL. Reduced expression of alpha5GABAA receptors elicits autism-like alterations in EEG patterns and sleep-wake behavior. Neurotoxicol Teratol. 2017;61:115–22.
Zurek AA, Kemp SW, Aga Z, Walker S, Milenkovic M, Ramsey AJ, et al. alpha5GABAA receptor deficiency causes autism-like behaviors. Ann Clin Transl Neurol. 2016;3:392–8.
Culiat CT, Stubbs LJ, Montgomery CS, Russell LB, Rinchik EM. Phenotypic consequences of deletion of the gamma 3, alpha 5, or beta 3 subunit of the type A gamma-aminobutyric acid receptor in mice. Proc Natl Acad Sci USA. 1994;91:2815–8.
DeLorey TM, Sahbaie P, Hashemi E, Homanics GE, Clark JD. Gabrb3 gene deficient mice exhibit impaired social and exploratory behaviors, deficits in non-selective attention and hypoplasia of cerebellar vermal lobules: a potential model of autism spectrum disorder. Behavioural Brain Res. 2008;187:207–20.
Homanics GE, DeLorey TM, Firestone LL, Quinlan JJ, Handforth A, Harrison NL, et al. Mice devoid of gamma-aminobutyrate type A receptor beta3 subunit have epilepsy, cleft palate, and hypersensitive behavior. Proc Natl Acad Sci USA. 1997;94:4143–8.
Gandhi T, Lee CC. Neural mechanisms underlying repetitive behaviors in rodent models of autism spectrum disorders. Front Cell Neurosci. 2020;14:592710.
Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, Fentress JC. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat Rev Neurosci. 2016;17:45–59.
Wu K, Castellano D, Tian Q, Lu W. Distinct regulation of tonic GABAergic inhibition by NMDA receptor subtypes. Cell Rep. 2021;37:109960.
Hughes RN. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Biobehav Rev. 2004;28:497–505.
Luscher B, Fuchs T, Kilpatrick CL. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70:385–409.
Castillo PE, Chiu CQ, Carroll RC. Long-term plasticity at inhibitory synapses. Curr Opin Neurobiol. 2011;21:328–38.
Marsden KC, Beattie JB, Friedenthal J, Carroll RC. NMDA receptor activation potentiates inhibitory transmission through GABA receptor-associated protein-dependent exocytosis of GABA(A) receptors. J Neurosci: Off J Soc Neurosci. 2007;27:14326–37.
Wiera G, Lebida K, Lech AM, Brzdak P, Van Hove I, De Groef L, et al. Long-term plasticity of inhibitory synapses in the hippocampus and spatial learning depends on matrix metalloproteinase 3. Cellular and molecular life sciences: CMLS. 2020.
Petrini EM, Ravasenga T, Hausrat TJ, Iurilli G, Olcese U, Racine V, et al. Synaptic recruitment of gephyrin regulates surface GABAA receptor dynamics for the expression of inhibitory LTP. Nat Commun. 2014;5:3921.
Angoa-Perez M, Kane MJ, Briggs DI, Francescutti DM, Kuhn DM. Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. J Vis Exp. 2013;82:50978.
Magnuson KM, Constantino JN. Characterization of depression in children with autism spectrum disorders. J Dev Behav Pediatr. 2011;32:332–40.
Gisbert Gustemps L, Lugo Marin J, Setien Ramos I, Ibanez Jimenez P, Romero Santo-Tomas O, Jurado Luque MJ, et al. Sleep disturbances in autism spectrum disorder without intellectual impairment: relationship with executive function and psychiatric symptoms. Sleep Med. 2021;83:106–14.
Mang GM, Nicod J, Emmenegger Y, Donohue KD, O’Hara BF, Franken P. Evaluation of a piezoelectric system as an alternative to electroencephalogram/ electromyogram recordings in mouse sleep studies. Sleep. 2014;37:1383–92.
Yaghouby F, Donohue KD, O’Hara BF, Sunderam S. Noninvasive dissection of mouse sleep using a piezoelectric motion sensor. J Neurosci Methods. 2016;259:90–100.
Davenport EC, Pendolino V, Kontou G, McGee TP, Sheehan DF, Lopez-Domenech G, et al. An essential role for the tetraspanin LHFPL4 in the cell-type-specific targeting and clustering of synaptic GABAA receptors. Cell Rep. 2017;21:70–83.
Yamasaki T, Hoyos-Ramirez E, Martenson JS, Morimoto-Tomita M, Tomita S. GARLH family proteins stabilize GABAA receptors at synapses. Neuron. 2017;93:1138–52 e6.
Ge Y, Kang Y, Cassidy RM, Moon KM, Lewis R, Wong ROL, et al. Clptm1 limits forward trafficking of GABAA receptors to scale inhibitory synaptic strength. Neuron. 2018;97:596–610 e8.
Diering GH, Huganir RL. The AMPA receptor code of synaptic plasticity. Neuron. 2018;100:314–29.
Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron. 2013;80:704–17.
Gu X, Zhou L, Lu W. An NMDA receptor-dependent mechanism underlies inhibitory synapse development. Cell Rep. 2016;14:471–8.
Horn ME, Nicoll RA. Somatostatin and parvalbumin inhibitory synapses onto hippocampal pyramidal neurons are regulated by distinct mechanisms. Proc Natl Acad Sci USA. 2018;115:589–94.
Gu X, Lu W. Genetic deletion of NMDA receptors suppresses GABAergic synaptic transmission in two distinct types of central neurons. Neurosci Lett. 2018;668:147–53.
Chiu CQ, Martenson JS, Yamazaki M, Natsume R, Sakimura K, Tomita S, et al. Input-Specific NMDAR-Dependent Potentiation of Dendritic GABAergic Inhibition. Neuron. 2018;97:368–77 e3.
Udakis M, Pedrosa V, Chamberlain SEL, Clopath C, Mellor JR. Interneuron-specific plasticity at parvalbumin and somatostatin inhibitory synapses onto CA1 pyramidal neurons shapes hippocampal output. Nat Commun. 2020;11:4395.
Braat S, Kooy RF. The GABAA receptor as a therapeutic target for neurodevelopmental disorders. Neuron. 2015;86:1119–30.
Brickley SG, Mody I. Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron. 2012;73:23–34.
Commons KG, Cholanians AB, Babb JA, Ehlinger DG. The Rodent forced swim test measures stress-coping strategy, not depression-like behavior. ACS Chem Neurosci. 2017;8:955–60.
Paul A, Crow M, Raudales R, He M, Gillis J, Huang ZJ. Transcriptional architecture of synaptic communication delineates GABAergic neuron identity. Cell. 2017;171:522–39 e20.
Zeisel A, Munoz-Manchado AB, Codeluppi S, Lonnerberg P, La Manno G, Jureus A, et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347:1138–42.
Acknowledgements
We are grateful to all members from the Lu laboratory for critical comments on the manuscript. We thank Daniel Abebe at NIH/NICHD for assistance with behavioral tests.
Funding
This work was supported by the NIH/NINDS Intramural Research Program (to WL), the NIH/NEI Intramural Research Program (to LD), Postdoctoral Fellowship from the NIH Center on Compulsive Behaviors (to RDS) and NINDS Diversity Training Fellowship (to DC).
Author information
Authors and Affiliations
Contributions
KW, RDS, and WL designed the project, and WL supervised the project. KW performed imaging and electrophysiological experiments. KW and WH performed biochemical experiments. KW and RDS performed behavioral assays. DC recorded GABA-evoked currents in HEK293T cells and neurons. QT performed neuronal cultures. LD generated Shisa7 S405A mutant mice. KW, RDS, and WL wrote the manuscript, and all authors read and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wu, K., Shepard, R.D., Castellano, D. et al. Shisa7 phosphorylation regulates GABAergic transmission and neurodevelopmental behaviors. Neuropsychopharmacol. 47, 2160–2170 (2022). https://doi.org/10.1038/s41386-022-01334-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-022-01334-0
This article is cited by
-
Restoration of Extrasynaptic/Synaptic GABAAR-α5 Localization Improves Sevoflurane-Induced Early Memory Impairment in Aged Mice
Neuroscience Bulletin (2026)
-
Music therapy as a preventive intervention for postpartum depression: modulation of synaptic plasticity, oxidative stress, and inflammation in a mouse model
Translational Psychiatry (2025)