Abstract
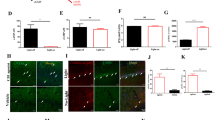
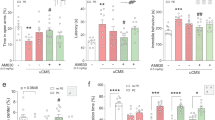
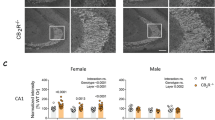
Peripheral inputs continuously shape brain function and can influence memory acquisition, but the underlying mechanisms have not been fully understood. Cannabinoid type-1 receptor (CB1R) is a well-recognized player in memory performance, and its systemic modulation significantly influences memory function. By assessing low arousal/non-emotional recognition memory in mice, we found a relevant role of peripheral CB1R in memory persistence. Indeed, the peripherally-restricted CB1R specific antagonist AM6545 showed significant mnemonic effects that were occluded in adrenalectomized mice, and after peripheral adrenergic blockade. AM6545 also transiently impaired contextual fear memory extinction. Vagus nerve chemogenetic inhibition reduced AM6545-induced mnemonic effect. Genetic CB1R deletion in dopamine β-hydroxylase-expressing cells enhanced recognition memory persistence. These observations support a role of peripheral CB1R modulating adrenergic tone relevant for cognition. Furthermore, AM6545 acutely improved brain connectivity and enhanced extracellular hippocampal norepinephrine. In agreement, intra-hippocampal β-adrenergic blockade prevented AM6545 mnemonic effects. Altogether, we disclose a novel CB1R-dependent peripheral mechanism with implications relevant for lengthening the duration of non-emotional memory.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Morris RGM. Elements of a neurobiological theory of hippocampal function: the role of synaptic plasticity, synaptic tagging and schemas. Eur J Neurosci. 2006;23:2829–46.
Kandel ER, Dudai Y, Mayford MR. The molecular and systems biology of memory. Cell 2014;157:163–86.
Yonelinas AP, Ranganath C, Ekstrom AD, Wiltgen BJ. A contextual binding theory of episodic memory: systems consolidation reconsidered. Nat Rev Neurosci. 2019;20:364–75.
De Quervain D, Schwabe L, Roozendaal B. Stress, glucocorticoids and memory: Implications for treating fear-related disorders. Nat Rev Neurosci. 2016;18:7–19.
Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–80.
Maccarrone M, Bab I, Bíró T, Cabral GA, Dey SK, Di Marzo V, et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharm Sci. 2015;36:277–96.
Pacher P, Bátkai S, Kunos G. The Endocannabinoid System as an emerging target of pharmacotherapy. Pharm Rev. 2006;58:389–462.
Castillo PE, Younts TJ, Chávez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron 2012;76:70–81.
Puighermanal E, Busquets-Garcia A, Maldonado R, Ozaita A. Cellular and intracellular mechanisms involved in the cognitive impairment of cannabinoids. Philos Trans R Soc Lond B Biol Sci. 2012;367:3254–63.
Niyuhire F, Varvel SA, Martin BR, Lichtman AH. Exposure to Marijuana smoke impairs memory retrieval in mice. J Pharm Exp Ther. 2007;322:1067–75.
Maccarrone M, Valverde O, Barbaccia ML, Castañé A, Maldonado R, Ledent C, et al. Age-related changes of anandamide metabolism in CB1 cannabinoid receptor knockout mice: correlation with behaviour. Eur J Neurosci. 2002;15:1178–86.
Reibaud M, Obinu MC, Ledent C, Parmentier M, Böhme GA, Imperato A. Enhancement of memory in cannabinoid CB1 receptor knock-out mice. Eur J Pharm. 1999;379:R1–2.
Zanettini C, Panlilio LV, Alicki M, Goldberg SR, Haller J, Yasar S. Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front Behav Neurosci. 2011;5:57.
Busquets-Garcia A, Gomis-González M, Srivastava RK, Cutando L, Ortega-Alvaro A, Ruehle S, et al. Peripheral and central CB1 cannabinoid receptors control stress-induced impairment of memory consolidation. Proc Natl Acad Sci USA. 2016;113:9904–9.
Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, NC3Rs Reporting Guidelines Working Group. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br J Pharm. 2010;160:1577–9.
Hillard CJ. Endocannabinoids and the endocrine system in health and disease. Handb Exp Pharm. 2015;231:317–39.
McIntyre CK, McGaugh JL, Williams CL. Interacting brain systems modulate memory consolidation. Neurosci Biobehav Rev. 2012;36:1750–62.
Introini-Collison I, Saghafi D, Novack GD, McGaugh JL. Memory-enhancing effects of post-training dipivefrin and epinephrine: involvement of peripheral and central adrenergic receptors. Brain Res. 1992;572:81–86.
Gold PE, Korol DL. Making memories matter. Front Integr Neurosci. 2012;6:116.
Robertson HT, Allison DB. Drugs associated with more suicidal ideations are also associated with more suicide attempts. PLoS One. 2009;4:e7312.
Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 2010;52:1059–69.
Cohen SJ, Stackman RW. Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav Brain Res. 2015;285:105–17.
Maroun M, Akirav I. Arousal and stress effects on consolidation and reconsolidation of recognition memory. Neuropsychopharmacology 2008 33:394–405.
Campolongo P, Morena M, Scaccianoce S, Trezza V, Chiarotti F, Schelling G, et al. Novelty-induced emotional arousal modulates cannabinoid effects on recognition memory and adrenocortical activity. Neuropsychopharmacology 2013;38:1276–86.
Puighermanal E, Marsicano G, Busquets-Garcia A, Lutz B, Maldonado R, Ozaita A. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci. 2009;12:1152–8.
Busquets-Garcia A, Puighermanal E, Pastor A, de la Torre R, Maldonado R, Ozaita A. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biol Psychiatry. 2011;70:479–86.
Takahashi RN, Pamplona FA, Fernandes MS. The cannabinoid antagonist SR141716A facilitates memory acquisition and consolidation in the mouse elevated T-maze. Neurosci Lett. 2005;380:270–5.
Lichtman AH. SR 141716A enhances spatial memory as assessed in a radial-arm maze task in rats. Eur J Pharm. 2000;404:175–9.
Wolff MC, Leander JD. SR141716A, a cannabinoid CB1 receptor antagonist, improves memory in a delayed radial maze task. Eur J Pharm. 2003;477:213–7.
Jacob W, Marsch R, Marsicano G, Lutz B, Wotjak CT. Cannabinoid CB1 receptor deficiency increases contextual fear memory under highly aversive conditions and long-term potentiation in vivo. Neurobiol Learn Mem. 2012;98:47–55.
Laricchiuta D, Balsamo F, Fabrizio C, Panuccio A, Termine A, Petrosini L. CB1 activity drives the selection of navigational strategies: A behavioral and c-fos immunoreactivity study. Int J Mol Sci. 2020;21:1072.
Ratano P, Everitt BJ, Milton AL. The CB1 Receptor Antagonist AM251 impairs reconsolidation of pavlovian fear memory in the rat basolateral amygdala. Neuropsychopharmacology. 2014;39:2529–37.
Lin HC, Mao SC, Gean PW. Effects of intra-amygdala infusion of CB1 receptor agonists on the reconsolidation of fear-potentiated startle. Learn Mem. 2006;13:316–21.
Santana F, Sierra RO, Haubrich J, Crestani AP, Duran JM, de Freitas Cassini L, et al. Involvement of the infralimbic cortex and CA1 hippocampal area in reconsolidation of a contextual fear memory through CB1 receptors: Effects of CP55,940. Neurobiol Learn Mem. 2016;127:42–7.
Tam J, Vemuri VK, Liu J, Bátkai S, Mukhopadhyay B, Godlewski G, et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 2010;120:2953–66.
Klumpers LE, Fridberg M, De Kam ML, Little PB, Jensen NO, Kleinloog HD, et al. Peripheral selectivity of the novel cannabinoid receptor antagonist TM38837 in healthy subjects. Br J Clin Pharmacol. 2013;76:846–57.
Flood JF, Smith GE, Morley JE. Modulation of memory processing by cholecystokinin: dependence on the vagus nerve. Science 1987;236:832–4.
Argueta DA, Perez PA, Makriyannis A, Di Patrizio NV. Cannabinoid CB1 receptors inhibit gut-brain satiation signaling in diet-induced obesity. Front Physiol. 2019;10:704.
Yang C, Liu J-F, Chai B-S, Fang Q, Chai N, Zhao L-Y, et al. Stress within a restricted time window selectively affects the persistence of long-term memory. PLoS One. 2013;8:e59075.
Roozendaal B, McGaugh JL. Memory modulation. Behav Neurosci. 2011;125:797–824.
Wade MR, Degroot A, Nomikos GG. Cannabinoid CB1 receptor antagonism modulates plasma corticosterone in rodents. Eur J Pharm. 2006;551:162–7.
Roberts CJ, Hillard CJ. Peripherally restricted cannabinoid type 1 receptor (CB1R) antagonist, AM6545, potentiates stress-induced hypothalamic–pituitary–adrenal axis activation via a non-CB1R mechanism. Endocrine. 2020;72:297–300.
Cluny NL, Vemuri VK, Chambers AP, Limebeer CL, Bedard H, Wood JT, et al. A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br J Pharm. 2010;161:629–42.
McReynolds JR, Christianson JP, Blacktop JM, Mantsch JR. What does the Fos say? Using Fos-based approaches to understand the contribution of stress to substance use disorders. Neurobiol Stress. 2018;9:271–85.
Silva BA, Burns AM, Gräff J. A cFos activation map of remote fear memory attenuation. Psychopharmacology. 2019;236:369–81.
Tanimizu T, Kono K, Kida S. Brain networks activated to form object recognition memory. Brain Res Bull. 2018;141:27–34.
Sala-Llonch R, Peña-Gómez C, Arenaza-Urquijo EM, Vidal-Piñeiro D, Bargalló N, Junqué C, et al. Brain connectivity during resting state and subsequent working memory task predicts behavioural performance. Cortex 2012;48:1187–96.
Kitamura T, Ogawa SK, Roy DS, Okuyama T, Morrissey MD, Smith LM, et al. Engrams and circuits crucial for systems consolidation of a memory. Science 2017;356:73–78.
Badran BW, Dowdle LT, Mithoefer OJ, LaBate NT, Coatsworth J, Brown JC, et al. Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: A concurrent taVNS/fMRI study and review. Brain Stimul. 2018;11;492–500.
Frangos E, Ellrich J, Komisaruk BR. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: FMRI evidence in humans. Brain Stimul. 2015;8:624–636.
Cao J, Lu KH, Powley TL, Liu Z. Vagal nerve stimulation triggers widespread responses and alters large-scale functional connectivity in the rat brain. PLoS One. 2017;12:e0189518.
Talley CE, Kahn S, Alexander LJ, Gold PE. Epinephrine fails to enhance performance of food-deprived rats on a delayed spontaneous alternation task. Neurobiol Learn Mem. 2000;73:79–86.
Dornelles A, de Lima MNM, Grazziotin M, Presti-Torres J, Garcia VA, Scalco FS, et al. Adrenergic enhancement of consolidation of object recognition memory. Neurobiol Learn Mem. 2007;88:137–42.
Bacon TJ, Pickering AE, Mellor JR. Noradrenaline release from locus coeruleus terminals in the hippocampus enhances excitation-spike coupling in ca1 pyramidal neurons via β-adrenoceptors. Cereb Cortex. 2020;30:6135–51.
Miyashita T, Williams C. Epinephrine administration increases neural impulses propagated along the vagus nerve: Role of peripheral β-adrenergic receptors. Neurobiol Learn Mem. 2006;85:116–24.
Vázquez-Oliver A, Brambilla-Pisoni C, Domingo-Gainza M, Maldonado R, Ivorra A, Ozaita A. Auricular transcutaneous vagus nerve stimulation improves memory persistence in naïve mice and in an intellectual disability mouse model. Brain Stimul. 2020;13:494–8.
Ghacibeh GA, Shenker JI, Shenal B, Uthman BM, Heilman KM. The influence of vagus nerve stimulation on memory. Cogn Behav Neurol. 2006;19:119–22.
Hansen N. The longevity of hippocampus-dependent memory is orchestrated by the locus coeruleus-noradrenergic System. Neural Plast. 2017;2017:1–9.
Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–23.
Hansen N, Manahan-Vaughan D. Locus coeruleus stimulation facilitates long-term depression in the dentate gyrus that requires activation of -adrenergic receptors. Cereb Cortex. 2015;25:1889–96.
Kemp A, Manahan-Vaughan D. Passive spatial perception facilitates the expression of persistent hippocampal long-term depression. Cereb Cortex. 2012;22:1614–21.
Kemp A, Manahan-Vaughan D. Adrenoreceptors comprise a critical element in learning-facilitated long-term plasticity. Cereb Cortex. 2008;18:1326–34.
Hagena H, Hansen N, Manahan-Vaughan D. β-adrenergic control of hippocampal function: subserving the choreography of synaptic information storage and Memory. Cereb Cortex. 2016;26:1349–64.
Acknowledgements
We thank Dulce Real, Marta Linares, and Francisco Porrón for expert technical assistance, and the Laboratory of Neuropharmacology-NeuroPhar for helpful discussion.
Funding
SMT was the recipient of a predoctoral fellowship (Generalitat de Catalunya) [FI-B00531 2016], ABM was supported by pre-doctoral fellowship (Generalitat de Catalunya) [FI_B0052 2020]. This study was supported by: Ministerio de Economía, Innovación y Competitividad (MINECO), Spain (#RTI2018-099282-B-I00B to AO), # PID2020-120029GB-I00 to RM; Generalitat de Catalunya, Spain (2017SGR-669 to RM); Basque Government, Spain (IT-1211-19 to JJM); ICREA (Institució Catalana de Recerca i Estudis Avançats, Spain) Academia to AO and RM. MELIS is the recipient of a grant “Unidad de Excelencia María de Maeztu”, funded by the MINECO (#MDM-2014-0370). FEDER, European Commission funding is also acknowledged. HDAC3-EAE-SCI Project with ref. PID2020-119769RA-I00 funded by MCIN/AEI/10.13039/501100011033 to AH. PRPDEVTAU Project with ref. PID2021-123714OB-I00 funded by MCINN/AEI/10.13039/501100011033/ and by “ERDF A way of making Europe” the CERCA Program, and the Commission for Universities and Research of the Department of Innovation, Universities, and Enterprise of the Generalitat de Catalunya (SGR2017-648) to JADR. Instituto de Salud Carlos III (ISCIII), “PI18/00893”, co-funded by the European Union, to GS. IBEC is the recipient of a Severo Ochoa Award of Excellence from MINECO (CEX2018-000789-S). SMT and AH were supported by Severo Ochoa program at IBEC. The authors have nothing to disclose.
Author information
Authors and Affiliations
Contributions
Behavioral, biochemical, histological, confocal and surgery experiments, statistical analyses and graphs and writing of the manuscript: SMT and ABM. Bilateral hippocampal cannula implantation in mice: LGL. Generation and provide the conditional transgenic mice (DBH-CB1KO): FR and BL Adrenalectomy in mice: AOA. Design and performance of the chemogenetic experiment: AH and JADR. Behavioral approaches: LdlRR. Chemogenetic surgeries in the vagus nerve: AH. rsfMRI experiments and conectomics on c-Fos-based network: EMM and GS. Microdialysis in vivo experiments in mice: JEO and JJM.. In vivo recordings in mice: JARO. Supervision of the study: RM. Conceptualization, supervision of the study, and writing of the manuscript: AO. All authors revised the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Martínez-Torres, S., Bergadà-Martínez, A., Ortega, J.E. et al. Peripheral CB1 receptor blockade acts as a memory enhancer through a noradrenergic mechanism. Neuropsychopharmacol. 48, 341–350 (2023). https://doi.org/10.1038/s41386-022-01436-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-022-01436-9