Abstract
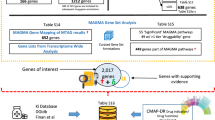
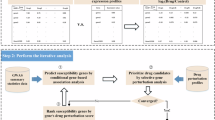
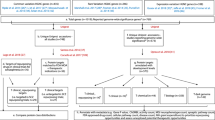
Psychiatric disorders impose tremendous economic burden on society and are leading causes of disability worldwide. However, only limited drugs are available for psychiatric disorders and the efficacy of most currently used drugs is poor for many patients. To identify novel therapeutic targets for psychiatric disorders, we performed genome-wide Mendelian randomization analyses by integrating brain-derived molecular quantitative trait loci (mRNA expression and protein abundance quantitative trait loci) of 1263 actionable proteins (targeted by approved drugs or drugs in clinical phase of development) and genetic findings from large-scale genome-wide association studies (GWASs). Using transcriptome data, we identified 25 potential drug targets for psychiatric disorders, including 12 genes for schizophrenia, 7 for bipolar disorder, 7 for depression, and 1 (TIE1) for attention deficit and hyperactivity. We also identified 10 actionable drug targets by using brain proteome data, including 4 (HLA-DRB1, CAMKK2, P2RX7, and MAPK3) for schizophrenia, 1 (PRKCB) for bipolar disorder, 6 (PSMB4, IMPDH2, SERPINC1, GRIA1, P2RX7 and TAOK3) for depression. Of note, MAPK3 and HLA-DRB1 were supported by both transcriptome and proteome-wide MR analyses, suggesting that these two proteins are promising therapeutic targets for schizophrenia. Our study shows the power of integrating large-scale GWAS findings and transcriptomic and proteomic data in identifying actionable drug targets. Besides, our findings prioritize actionable novel drug targets for development of new therapeutics and provide critical drug-repurposing opportunities for psychiatric disorders.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
GBD. 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789–858.
Vigo D, Thornicroft G, Atun R. Estimating the true global burden of mental illness. Lancet Psychiatry. 2016;3:171–8.
Steel Z, Marnane C, Iranpour C, Chey T, Jackson JW, Patel V, et al. The global prevalence of common mental disorders: a systematic review and meta-analysis 1980–2013. Int J Epidemiol. 2014;43:476–93.
Trautmann S, Rehm J, Wittchen HU. The economic costs of mental disorders: Do our societies react appropriately to the burden of mental disorders? EMBO Rep. 2016;17:1245–9.
COVID-19 Mental Disorders Collaborators*. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021; https://doi.org/10.1016/S0140-6736(21)02143-7.
Miyamoto S, Miyake N, Jarskog LF, Fleischhacker WW, Lieberman JA. Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol Psychiatry. 2012;17:1206–27.
Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104.
Patel KR, Cherian J, Gohil K, Atkinson D. Schizophrenia: overview and treatment options. P t. 2014;39:638–45.
Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet 2012;379:1045–55.
Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 2018;391:1357–66.
Buckley PF. Update on the treatment and management of schizophrenia and bipolar disorder. CNS Spectr. 2008;13:1–10.
Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, et al. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13:318–78.
Carlsson A, Lindqvist M. Effect of chlorpromazine or Haloperidol on Formation of 3Methoxytyramine and Normetanephrine in Mouse Brain. Acta Pharm Toxicol (Copenh). 1963;20:140–4.
van Rossum JM. The significance of dopamine-receptor blockade for the mechanism of action of neuroleptic drugs. Arch Int Pharmacodyn Ther. 1966;160:492–4.
Seeman P. Atypical antipsychotics: mechanism of action. Can J Psychiatry. 2002;47:27–38.
Taylor C, Fricker AD, Devi LA, Gomes I. Mechanisms of action of antidepressants: from neurotransmitter systems to signaling pathways. Cell Signal. 2005;17:549–57.
Brigitta B. Pathophysiology of depression and mechanisms of treatment. Dialogues Clin Neurosci. 2002;4:7–20.
Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, et al. Major depressive disorder. Nat Rev Dis Prim. 2016;2:16065.
Uçok A, Gaebel W. Side effects of atypical antipsychotics: a brief overview. World Psychiatry. 2008;7:58–62.
Muench J, Hamer AM. Adverse effects of antipsychotic medications. Am Fam Physician. 2010;81:617–22.
Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry. 2018;17:341–56.
Uher R, Farmer A, Henigsberg N, Rietschel M, Mors O, Maier W, et al. Adverse reactions to antidepressants. Br J Psychiatry. 2009;195:202–10.
Warnock JK, Knesevich JW. Adverse cutaneous reactions to antidepressants. Am J Psychiatry. 1988;145:425–30.
Schmidt LG, Grohmann R, Müller-Oerlinghausen B, Ochsenfahrt H, Schönhöfer PS. Adverse drug reactions to first- and second-generation antidepressants: a critical evaluation of drug surveillance data. Br J Psychiatry. 1986;148:38–43.
Santarsieri D, Schwartz TL. Antidepressant efficacy and side-effect burden: a quick guide for clinicians. Drugs Context. 2015;4:212290.
Khawam EA, Laurencic G, Malone DA Jr. Side effects of antidepressants: an overview. Cleve Clin J Med. 2006;73:351–3.
Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet 2019;394:939–51.
Suzuki T, Remington G, Mulsant BH, Uchida H, Rajji TK, Graff-Guerrero A, et al. Defining treatment-resistant schizophrenia and response to antipsychotics: a review and recommendation. Psychiatry Res. 2012;197:1–6.
Correll CU, Howes OD. Treatment-Resistant Schizophrenia: Definition, Predictors, and Therapy Options. J Clin Psychiatry. 2021;82:MY20096AH1C.
Siskind D, Orr S, Sinha S, Yu Q, Brijball B, Warren N, et al. Rates of treatment-resistant schizophrenia from dirst-episode cohorts: systematic review and meta-analysis. The British Journal of Psychiatry. 2021; https://doi.org/10.1192/bjp.2021.61.
Nucifora FC Jr, Woznica E, Lee BJ, Cascella N, Sawa A. Treatment resistant schizophrenia: Clinical, biological, and therapeutic perspectives. Neurobiol Dis. 2019;131:104257.
Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369–88.
Howes OD, Thase ME, Pillinger T Treatment resistance in psychiatry: state of the art and new directions. Mol Psychiatry. 2021; https://doi.org/10.1038/s41380-021-01200-3.
Little JT, Reynolds CF 3rd, Dew MA, Frank E, Begley AE, Miller MD, et al. How common is resistance to treatment in recurrent, nonpsychotic geriatric depression? Am J Psychiatry. 1998;155:1035–8.
Agid Y, Buzsáki G, Diamond DM, Frackowiak R, Giedd J, Girault JA, et al. How can drug discovery for psychiatric disorders be improved? Nat Rev Drug Disco. 2007;6:189–201.
Editorials. Psychiatric drug discovery on the couch. Nat Rev Drug Disco. 2007;6:171.
Zamponi GW. Targeting voltage-gated calcium channels in neurological and psychiatric diseases. Nat Rev Drug Disco. 2016;15:19–34.
Kesselheim AS, Hwang TJ, Franklin JM. Two decades of new drug development for central nervous system disorders. Nat Rev Drug Disco. 2015;14:815–6.
Reay WR, Cairns MJ. Advancing the use of genome-wide association studies for drug repurposing. Nat Rev Genet. 2021;22:658–71.
Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014;511:421–7.
Lam M, Chen CY, Li Z, Martin AR, Bryois J, Ma X, et al. Comparative genetic architectures of schizophrenia in East Asian and European populations. Nat Genet. 2019;51:1670–8.
Shi Y, Li Z, Xu Q, Wang T, Li T, Shen J, et al. Common variants on 8p12 and 1q24.2 confer risk of schizophrenia. Nat Genet. 2011;43:1224–7.
Yue WH, Wang HF, Sun LD, Tang FL, Liu ZH, Zhang HX, et al. Genome-wide association study identifies a susceptibility locus for schizophrenia in Han Chinese at 11p11.2. Nat Genet. 2011;43:1228–31.
Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50:668–81.
Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 2019;22:343–52.
Mullins N, Forstner AJ, O’Connell KS, Coombes B, Coleman JRI, Qiao Z, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. 2021;53:817–29.
Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. 2019;51:63–75.
Nelson MR, Tipney H, Painter JL, Shen J, Nicoletti P, Shen Y, et al. The support of human genetic evidence for approved drug indications. Nat Genet. 2015;47:856–60.
Plenge RM, Scolnick EM, Altshuler D. Validating therapeutic targets through human genetics. Nat Rev Drug Disco. 2013;12:581–94.
Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl J Med. 2006;354:1264–72.
Lopalco L. CCR5: From Natural Resistance to a New Anti-HIV Strategy. Viruses 2010;2:574–600.
Hindorff LA, Sethupathy P, Junkins HA, Ramos EM, Mehta JP, Collins FS, et al. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci. 2009;106:9362–7.
Gusev A, Ko A, Shi H, Bhatia G, Chung W, Penninx BW, et al. Integrative approaches for large-scale transcriptome-wide association studies. Nat Genet. 2016;48:245–52.
Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23:R89–98.
Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. Bmj 2018;362:k601.
Holmes MV, Ala-Korpela M, Smith GD. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nat Rev Cardiol. 2017;14:577–90.
Ference BA, Robinson JG, Brook RD, Catapano AL, Chapman MJ, Neff DR, et al. Variation in PCSK9 and HMGCR and Risk of Cardiovascular Disease and Diabetes. N. Engl J Med. 2016;375:2144–53.
Swerdlow DI, Holmes MV, Kuchenbaecker KB, Engmann JE, Shah T, Sofat R, et al. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet 2012;379:1214–24.
Larsson SC, Traylor M, Malik R, Dichgans M, Burgess S, Markus HS. Modifiable pathways in Alzheimer’s disease: Mendelian randomisation analysis. Bmj 2017;359:j5375.
Gaziano L, Giambartolomei C, Pereira AC, Gaulton A, Posner DC, Swanson SA, et al. Actionable druggable genome-wide Mendelian randomization identifies repurposing opportunities for COVID-19. Nat Med. 2021;27:668–76.
Mendez D, Gaulton A, Bento AP, Chambers J, De Veij M, Félix E, et al. ChEMBL: towards direct deposition of bioassay data. Nucleic Acids Res. 2019;47:D930–d40.
GTEx Consortium. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 2015;348:648–60.
Wang D, Liu S, Warrell J, Won H, Shi X, Navarro FCP, et al. Comprehensive functional genomic resource and integrative model for the human brain. Science. 2018;362:eaat8464.
Wingo AP, Liu Y, Gerasimov ES, Gockley J, Logsdon BA, Duong DM, et al. Integrating human brain proteomes with genome-wide association data implicates new proteins in Alzheimer’s disease pathogenesis. Nat Genet. 2021;53:143–6.
Liu A, Manuel AM, Dai Y, Zhao Z. Prioritization of risk genes in multiple sclerosis by a refined Bayesian framework followed by tissue-specificity and cell type feature assessment. BMC Genomics. 2022;23:362.
Richardson TG, Hemani G, Gaunt TR, Relton CL, Davey, Smith G. A transcriptome-wide Mendelian randomization study to uncover tissue-dependent regulatory mechanisms across the human phenome. Nat Commun. 2020;11:185.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408.
Zhu Z, Zhang F, Hu H, Bakshi A, Robinson MR, Powell JE, et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat Genet. 2016;48:481–7.
Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, et al. A global reference for human genetic variation. Nature 2015;526:68–74.
Elsworth B, Lyon M, Alexander T, Liu Y, Matthews P, Hallett J, et al. The MRC IEU OpenGWAS data infrastructure. bioRxiv. 2020.
Deng YT, Ou YN, Wu BS, Yang YX, Jiang Y, Huang YY, et al. Identifying causal genes for depression via integration of the proteome and transcriptome from brain and blood. Mol Psychiatry. 2022;27:2849–57.
Liu J, Li M, Luo XJ, Su B. Systems-level analysis of risk genes reveals the modular nature of schizophrenia. Schizophr Res. 2018;201:261–9.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504.
Polioudakis D, de la Torre-Ubieta L, Langerman J, Elkins AG, Shi X, Stein JL, et al. A Single-Cell Transcriptomic Atlas of Human Neocortical Development during Mid-gestation. Neuron 2019;103:785–801.e8.
Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419.
Liu J, Li X, Luo XJ. Proteome-wide Association Study Provides Insights Into the Genetic Component of Protein Abundance in Psychiatric Disorders. Biol Psychiatry. 2021;90:781–9.
Berger SM, Bartsch D. The role of L-type voltage-gated calcium channels Cav1.2 and Cav1.3 in normal and pathological brain function. Cell Tissue Res. 2014;357:463–76.
Moon AL, Haan N, Wilkinson LS, Thomas KL, Hall J. CACNA1C: Association With Psychiatric Disorders, Behavior, and Neurogenesis. Schizophr Bull. 2018;44:958–65.
Föcking M, Lopez LM, English JA, Dicker P, Wolff A, Brindley E, et al. Proteomic and genomic evidence implicates the postsynaptic density in schizophrenia. Mol Psychiatry. 2015;20:424–32.
Gusev A, Mancuso N, Won H, Kousi M, Finucane HK, Reshef Y, et al. Transcriptome-wide association study of schizophrenia and chromatin activity yields mechanistic disease insights. Nat Genet. 2018;50:538–48.
Ma C, Gu C, Huo Y, Li X, Luo XJ. The integrated landscape of causal genes and pathways in schizophrenia. Transl Psychiatry. 2018;8:67.
Chang H, Cai X, Li HJ, Liu WP, Zhao LJ, Zhang CY, et al. Functional Genomics Identify a Regulatory Risk Variation rs4420550 in the 16p11.2 Schizophrenia-Associated Locus. Biol Psychiatry. 2021;89:246–55.
Wong ML, Dong C, Maestre-Mesa J, Licinio J. Polymorphisms in inflammation-related genes are associated with susceptibility to major depression and antidepressant response. Mol Psychiatry. 2008;13:800–12.
Kapfhamer D, King I, Zou ME, Lim JP, Heberlein U, Wolf FW. JNK pathway activation is controlled by Tao/TAOK3 to modulate ethanol sensitivity. PLoS One. 2012;7:e50594.
Li Z, Oh H, Cung M, Marquez SJ, Sun J, Hammad H, et al. TAOK3 is a MAP3K contributing to osteoblast differentiation and skeletal mineralization. Biochem Biophys Res Commun. 2020;531:497–502.
Cheng SW, Li JX, Chien YC, Chang JP, Shityakov S, Huang SY, et al. Genetic Variations of Ionotropic Glutamate Receptor Pathways on Interferon-alpha-induced Depression in Patients with Hepatitis C Viral Infection. Brain Behav Immun. 2021;93:16–22.
Fang K, Xu JX, Chen XX, Gao XR, Huang LL, Du AQ, et al. Differential serum exosome microRNA profile in a stress-induced depression rat model. J Affect Disord. 2020;274:144–58.
Humo M, Ayazgok B, Becker LJ, Waltisperger E, Rantamaki T, Yalcin I. Ketamine induces rapid and sustained antidepressant-like effects in chronic pain induced depression: Role of MAPK signaling pathway. Prog Neuropsychopharmacol Biol Psychiatry. 2020;100:109898.
Wang JQ, Mao L. The ERK Pathway: Molecular Mechanisms and Treatment of Depression. Mol Neurobiol. 2019;56:6197–205.
Liao C, Laporte AD, Spiegelman D, Akçimen F, Joober R, Dion PA, et al. Transcriptome-wide association study of attention deficit hyperactivity disorder identifies associated genes and phenotypes. Nat Commun. 2019;10:4450.
Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–23.
Franke B, Faraone SV, Asherson P, Buitelaar J, Bau CH, Ramos-Quiroga JA, et al. The genetics of attention deficit/hyperactivity disorder in adults, a review. Mol Psychiatry. 2012;17:960–87.
Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–92.
Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–62.
Ghazalpour A, Bennett B, Petyuk VA, Orozco L, Hagopian R, Mungrue IN, et al. Comparative analysis of proteome and transcriptome variation in mouse. PLoS Genet. 2011;7:e1001393.
Wingo TS, Liu Y, Gerasimov ES, Gockley J, Logsdon BA, Duong DM, et al. Brain proteome-wide association study implicates novel proteins in depression pathogenesis. Nat Neurosci. 2021;24:810–7.
Dall’Aglio L, Lewis CM, Pain O. Delineating the Genetic Component of Gene Expression in Major Depression. Biol Psychiatry. 2021;89:627–36.
Kibinge NK, Relton CL, Gaunt TR, Richardson TG. Characterizing the Causal Pathway for Genetic Variants Associated with Neurological Phenotypes Using Human Brain-Derived Proteome Data. Am J Hum Genet. 2020;106:885–92.
Hall LS, Medway CW, Pain O, Pardiñas AF, Rees EG, Escott-Price V, et al. A transcriptome-wide association study implicates specific pre- and post-synaptic abnormalities in schizophrenia. Hum Mol Genet. 2020;29:159–67.
Baird DA, Liu JZ, Zheng J, Sieberts SK, Perumal T, Elsworth B, et al. Identifying drug targets for neurological and psychiatric disease via genetics and the brain transcriptome. PLoS Genet. 2021;17:e1009224.
Png G, Barysenka A, Repetto L, Navarro P, Shen X, Pietzner M, et al. Mapping the serum proteome to neurological diseases using whole genome sequencing. Nat Commun. 2021;12:7042.
Zeng B, Bendl J, Kosoy R, Fullard JF, Hoffman GE, Roussos P. Multi-ancestry eQTL meta-analysis of human brain identifies candidate causal variants for brain-related traits. Nat Genet. 2022;54:161–9.
Acknowledgements
We thank Miss. Qian Li for her technical assistance.
Funding
This study was equally supported by the Distinguished Young Scientists grant of the Yunnan Province (202001AV070006) and the Key Research Project of Yunnan Province (202101AS070055 to XJL). Also was supported by the National Nature Science Foundation of China (U2102205 and 31970561 to XJL), the CAS “Light of West China” Program (to JWL), the Yunnan Fundamental Research Projects (202001AT070099 to JWL), and the National Natural Science Key Foundation of China (No. 81830040 and 82130042 to ZJZ).
Author information
Authors and Affiliations
Contributions
XJL conceived, designed and supervised the whole study. JWL performed the analyses. XJL, JWL, QYC, ML, ZJZ and TL wrote the manuscript. All authors provided critical comments and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, J., Cheng, Y., Li, M. et al. Genome-wide Mendelian randomization identifies actionable novel drug targets for psychiatric disorders. Neuropsychopharmacol. 48, 270–280 (2023). https://doi.org/10.1038/s41386-022-01456-5
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41386-022-01456-5
This article is cited by
-
Cross-ancestry genome-wide association study and systems-level integrative analyses implicate new risk genes and therapeutic targets for depression
Nature Human Behaviour (2025)
-
Leveraging pleiotropy for the improved treatment of psychiatric disorders
Molecular Psychiatry (2025)
-
PSMB4: a potential biomarker and therapeutic target for depression, perspective from integration analysis of depression GWAS data and human plasma proteome
Translational Psychiatry (2025)
-
Novel drug targets for psoriasis identified through Mendelian randomization study
Archives of Dermatological Research (2025)
-
Identification of Mood Disorders Causal Genes by Integrating the Brain Proteome and Transcriptome
Molecular Neurobiology (2025)