Abstract
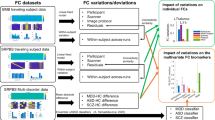
Substantial clinical heterogeneity and comorbidity inherent amongst mental disorders limit the identification of neuroimaging biomarkers that can reliably track clinical symptoms. Strategies that enable generation of meaningful and replicable neurobiological markers at the individual level will push the field of neuropsychiatry forward in developing efficacious personalized treatment. The current study included 142 adult patients with a primary diagnosis of schizophrenia (SCZ), bipolar (BP), or attention deficit/hyperactivity disorder (ADHD), and 67 patient ratings across four behavioral measures. Using functional connectivity derived from a personalized fMRI approach, we identified several candidate imaging markers related to dimensional phenotypes across disorders, assessed the internal and external generalizability of these markers, and compared the probability of replicating findings across datasets using individual and group-averaged defined functional regions. We identified subject-specific connections related to three different clinical domains (attention deficit, appetite-energy, psychosis-positive) in a discovery dataset. Importantly, these connectivity biomarkers were robust and were reproduced in an independent validation dataset. For markers related to neurovegetative symptoms (attention deficit, appetite-energy symptoms), the brain connections involved showed similar connectivity patterns across the different diagnoses. However, psychosis-positive symptoms were associated with connections of varying strength across disorders. Finally, we found that markers for symptom domains were replicable for individually-specified connections, but not for group template-derived connections. Our personalized strategies allowed us to identify meaningful and generalizable imaging markers for symptom domains in patients who exhibit high levels of heterogeneity. These biomarkers may shed new light on the connectivity underpinnings of psychiatric symptoms and lead to personalized interventions.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neurosci Biobehav Rev. 2011;35:1110–24.
Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–84.
Cole MW, Anticevic A, Repovs G, Barch D. Variable global dysconnectivity and individual differences in schizophrenia. Biol Psychiatry. 2011;70:43–50.
Downar J, Blumberger DM, Daskalakis ZJ. The neural crossroads of psychiatric illness: an emerging target for brain stimulation. Trends Cogn Sci. 2016;20:107–20.
Wang D, Li M, Wang M, Schoeppe F, Ren J, Chen H, et al. Individual-specific functional connectivity markers track dimensional and categorical features of psychotic illness. Mol Psychiatry. 2020;25:2119–29.
Craddock N, Owen MJ. Rethinking psychosis: the disadvantages of a dichotomous classification now outweigh the advantages. World Psychiatry. 2007;6:84–91.
Bilder RM, Sabb FW, Cannon TD, London ED, Jentsch JD, Parker DS, et al. Phenomics: the systematic study of phenotypes on a genome-wide scale. Neuroscience. 2009;164:30–42.
Mueller S, Wang D, Fox MD, Yeo BT, Sepulcre J, Sabuncu MR, et al. Individual variability in functional connectivity architecture of the human brain. Neuron. 2013;77:586–95.
Gordon EM, Laumann TO, Gilmore AW, Newbold DJ, Greene DJ, Berg JJ, et al. Precision functional mapping of individual human brains. Neuron. 2017;95:791–807.e7.
Li M, Wang D, Ren J, Langs G, Stoecklein S, Brennan BP, et al. Performing group-level functional image analyses based on homologous functional regions mapped in individuals. PLoS Biol. 2019;17:e2007032.
Lauren AM, Lebois, Li M, Baker JT, Wolff JD, Wang D, et al. Large-scale functional brain network architecture changes associated with trauma-related dissociation. Am J Psychiatry. 2020;0:19060647.
Poldrack RA, Huckins G, Varoquaux G. Establishment of best practices for evidence for prediction: a review. JAMA Psychiatry. 2020;77:534–40.
Poldrack RA, Congdon E, Triplett W, Gorgolewski KJ, Karlsgodt KH, Mumford JA, et al. A phenome-wide examination of neural and cognitive function. Sci Data. 2016;3:160110.
Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–5.
Li M, Dahmani L, Wang D, Ren J, Stocklein S, Lin Y, et al. Co-activation patterns across multiple tasks reveal robust anti-correlated functional networks. NeuroImage. 2021;227:117680.
Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014;83:238–51.
Chen RH, Ito T, Kulkarni KR, Cole MW. The human brain traverses a common activation-pattern state space across task and rest. Brain Connect. 2018;8:429–43.
Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65.
Murtagh F, Legendre P. Ward’s hierarchical agglomerative clustering method: which algorithms implement Ward’s criterion? J Classif. 2014;31:274–95.
Carter JD, Bizzell J, Kim C, Bellion C, Carpenter KL, Dichter G, et al. Attention deficits in schizophrenia–preliminary evidence of dissociable transient and sustained deficits. Schizophr Res. 2010;122:104–12.
Katzman MA, Bilkey TS, Chokka PR, Fallu A, Klassen LJ. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17:302.
Robinson LJ, Ferrier IN. Evolution of cognitive impairment in bipolar disorder: a systematic review of cross-sectional evidence. Bipolar Disord. 2006;8:103–16.
Vasterling JJ, Brailey K, Constans JI, Sutker PB. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12:125–33.
Posner MI, Rothbart MK, Vizueta N, Levy KN, Evans DE, Thomas KM, et al. Attentional mechanisms of borderline personality disorder. Proc Natl Acad Sci USA. 2002;99:16366–70.
Sha Z, Wager TD, Mechelli A, He Y. Common dysfunction of large-scale neurocognitive networks across psychiatric disorders. Biol Psychiatry. 2019;85:379–88.
Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89.
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56.
Uddin LQ, Nomi JS, Hébert-Seropian B, Ghaziri J, Boucher O. Structure and function of the human insula. J Clin Neurophysiol. 2017;34:300–6.
Lis M, Stańczykiewicz B, Liśkiewicz P, Misiak B. Impaired hormonal regulation of appetite in schizophrenia: a narrative review dissecting intrinsic mechanisms and the effects of antipsychotics. Psychoneuroendocrinology. 2020;119:104744.
Platzer M, Fellendorf FT, Bengesser SA, Birner A, Dalkner N, Hamm C, et al. The relationship between food craving, appetite-related hormones and clinical parameters in bipolar disorder. Nutrients. 2020;13:76.
Simmons WK, Burrows K, Avery JA, Kerr KL, Bodurka J, Savage CR, et al. Depression-related increases and decreases in appetite: dissociable patterns of aberrant activity in reward and interoceptive neurocircuitry. Am J Psychiatry. 2016;173:418–28.
Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702.
Diler RS, Daviss WB, Lopez A, Axelson D, Iyengar S, Birmaher B. Differentiating major depressive disorder in youths with attention deficit hyperactivity disorder. J Affect Disord. 2007;102:125–30.
Weiss M, Worling D, Wasdell M. A chart review study of the inattentive and combined types of ADHD. J Atten Disord. 2003;7:1–9.
Burrows T, Kay-Lambkin F, Pursey K, Skinner J, Dayas C. Food addiction and associations with mental health symptoms: a systematic review with meta-analysis. J Hum Nutr Dietetics. 2018;31:544–72.
Lamme VA, Supèr H, Spekreijse H. Feedforward, horizontal, and feedback processing in the visual cortex. Curr Opin Neurobiol. 1998;8:529–35.
Yamashita A, Yahata N, Itahashi T, Lisi G, Yamada T, Ichikawa N, et al. Harmonization of resting-state functional MRI data across multiple imaging sites via the separation of site differences into sampling bias and measurement bias. PLoS Biol. 2019;17:e3000042.
Fortin JP, Parker D, Tunç B, Watanabe T, Elliott MA, Ruparel K, et al. Harmonization of multi-site diffusion tensor imaging data. Neuroimage. 2017;161:149–70.
Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38.
Boeke EA, Holmes AJ, Phelps EA. Toward robust anxiety biomarkers: a machine learning approach in a large-scale sample. Biol Psychiatry: Cogn Neurosci Neuroimaging. 2020;5:799–807.
Jin D, Zhou B, Han Y, Ren J, Han T, Liu B, et al. Generalizable, reproducible, and neuroscientifically interpretable imaging biomarkers for Alzheimer’s disease. Adv Sci. 2020;7:2000675.
Gordon EM, Laumann TO, Adeyemo B, Petersen SE. Individual variability of the system-level organization of the human brain. Cereb Cortex. 2017;27:386–99.
Funding
This work was supported by Changping Laboratory and the Ministry of Science and Technology of China (2021B-01-01), National Natural Science Foundation of China grants Nos. 81790652, 81790650, and NIH grants P50MH106435, 1R01DC017991, 5K01MH111802. LD is supported by a Canadian Institutes of Health Research postdoctoral fellowship, FRN: MFE-171291. These data were obtained from the OpenfMRI database ds000030, funded by Consortium for Neuropsychiatric Phenomics (NIH Roadmap for Medical Research grants).
Author information
Authors and Affiliations
Contributions
ML and HL conceived the study; ML and LD performed the analyses with support from YH and HL; ML, LD, DW, MW, CSH and HL wrote the manuscript. All authors commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, M., Dahmani, L., Hubbard, C.S. et al. Individualized functional connectome identified generalizable biomarkers for psychiatric symptoms in transdiagnostic patients. Neuropsychopharmacol. 48, 633–641 (2023). https://doi.org/10.1038/s41386-022-01500-4
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41386-022-01500-4
This article is cited by
-
Connectome-based predictive modeling of early and chronic psychosis symptoms
Neuropsychopharmacology (2025)
-
Human lifespan changes in the brain’s functional connectome
Nature Neuroscience (2025)