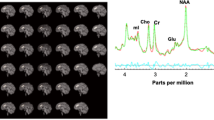
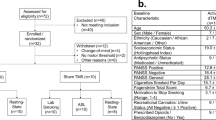
Abstract
The cholinergic system is a critical mediator of cognition in animals. People who smoke cigarettes exhibit cognitive deficits, especially during quit attempts. Few studies jointly examine the cholinergic system and cognition in people while trying to quit smoking. We used positron emission tomography (PET) brain imaging with the β2-subunit containing nicotinic acetylcholine receptor (β2*-nAChR) partial agonist radioligand (-)-[18F]flubatine and the acetylcholinesterase inhibitor physostigmine to jointly examine the cholinergic system, smoking status, and cognition. (-)-[18F]Flubatine scans and cognitive data were acquired from twenty people who recently stopped smoking cigarettes (aged 38 ± 11 years; 6 female, 14 male; abstinent 7 ± 1 days) and 27 people who never smoked cigarettes (aged 29 ± 8 years; 11 female, 16 male). A subset of fifteen recently abstinent smokers and 21 never smokers received a mid-scan physostigmine challenge to increase acetylcholine levels. Regional volume of distribution (VT) was estimated with equilibrium analysis at “baseline” and post-physostigmine. Participants completed a cognitive battery prior to (-)-[18F]flubatine injection and physostigmine administration assessing executive function (Groton Maze Learning test), verbal learning (International Shopping List test), and working memory (One Back test). Physostigmine significantly decreased cortical (-)-[18F]flubatine VT, consistent with increased cortical acetylcholine levels reducing the number of β2*-nAChR sites available for (-)-[18F]flubatine binding, at comparable magnitudes across groups (p values < 0.05). A larger magnitude of physostigmine-induced decrease in (-)-[18F]flubatine VT was significantly associated with worse executive function in people who recently stopped smoking (p values < 0.05). These findings underscore the role of the cholinergic system in early smoking cessation and highlight the importance of neuroscience-informed treatment strategies.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
West R. Tobacco smoking: health impact, prevalence, correlates and interventions. Psychol Health. 2017;32:1018–36.
Creamer M, Wang T, Babb S, Cullen K, Day H, Willis G, et al. Tobacco product use and cessation indicators among adults—United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:1013–9.
Leventhal AM, Waters AJ, Moolchan ET, Heishman SJ, Pickworth WB. A quantitative analysis of subjective, cognitive, and physiological manifestations of the acute tobacco abstinence syndrome. Addict Behav. 2010;35:1120–30.
Powell JH, Pickering AD, Dawkins L, West R, Powell JF. Cognitive and psychological correlates of smoking abstinence, and predictors of successful cessation. Addict Behav. 2004;29:1407–26.
Ashare RL, Falcone M, Lerman C. Cognitive function during nicotine withdrawal: Implications for nicotine dependence treatment. Neuropharmacology 2014;76:581–91.
Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, et al. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug Alcohol Depend. 2010;106:61–4.
Valentine G, Sofuoglu M. Cognitive effects of nicotine: recent progress. Curr Neuropharmacol. 2018;16:403–14.
Conti AA, McLean L, Tolomeo S, Steele JD, Baldacchino A. Chronic tobacco smoking and neuropsychological impairments: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2019;96:143–54.
Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–7.
Friedman NP, Robbins TW. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology 2022;47:72–89.
Badre D, Nee DE. Frontal cortex and the hierarchical control of behavior. Trends Cogn Sci. 2018;22:170–88.
Jasinska AJ, Zorick T, Brody AL, Stein EA. Dual role of nicotine in addiction and cognition: a review of neuroimaging studies in humans. Neuropharmacology. 2014;84:111–22.
Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, et al. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173.
Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature 1996;382:255–7.
Imperato A, Mulas A, Di Chiara G. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur J Pharm. 1986;132:337–8.
Wills L, Kenny PJ. Addiction-related neuroadaptations following chronic nicotine exposure. J Neurochem. 2021;157:1652–73.
Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: Activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–42.
Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76:116–29.
Nees F. The nicotinic cholinergic system function in the human brain. Neuropharmacology. 2015;96:289–301.
Mukhin AG, Kimes AS, Chefer SI, Matochik JA, Contoreggi CS, Horti AG, et al. Greater nicotinic acetylcholine receptor density in smokers than in nonsmokers: a PET study with 2-18F-FA-85380. J Nucl Med. 2008;49:1628–35.
Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, et al. Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. J Neurosci. 2006;26:8707.
Cosgrove KP, Batis J, Bois F, Maciejewski PK, Esterlis I, Kloczynski T, et al. β2-nicotinic acetylcholine receptor availability during acute and prolonged abstinence from tobacco smoking. JAMA Psychiatry. 2009;66:666–76.
Wüllner U, Gündisch D, Herzog H, Minnerop M, Joe A, Warnecke M, et al. Smoking upregulates α4β2* nicotinic acetylcholine receptors in the human brain. Neurosci Lett. 2008;430:34–7.
Mamede M, Ishizu K, Ueda M, Mukai T, Iida Y, Kawashima H, et al. Temporal change in human nicotinic acetylcholine receptor after smoking cessation: 5IA SPECT study. J Nucl Med. 2007;48:1829–35.
Brody AL, Mukhin AG, Mamoun MS, Luu T, Neary M, Liang L, et al. Brain nicotinic acetylcholine receptor availability and response to smoking cessation treatment: a randomized trial. JAMA Psychiatry. 2014;71:797–805.
Brody AL, Mukhin AG, Stephanie S, Mamoun MS, Kozman M, Phuong J, et al. Treatment for Tobacco Dependence: Effect on Brain Nicotinic Acetylcholine Receptor Density. Neuropsychopharmacology. 2013;38:1548–56.
Hillmer AT, Esterlis I, Gallezot JD, Bois F, Zheng MQ, Nabulsi N, et al. Imaging of cerebral α4β2* nicotinic acetylcholine receptors with (−)-[18F]Flubatine PET: Implementation of bolus plus constant infusion and sensitivity to acetylcholine in human brain. Neuroimage. 2016;141:71–80.
Esterlis I, Hannestad JO, Bois F, Sewell RA, Tyndale RF, Seibyl JP, et al. Imaging changes in synaptic acetylcholine availability in living human subjects. J Nucl Med. 2013;54:78–82.
Gallezot J-D, Esterlis I, Bois F, Zheng M-Q, Lin S-F, Kloczynski T, et al. Evaluation of the sensitivity of the novel α4β2* nicotinic acetylcholine receptor PET radioligand 18F-(-)-NCFHEB to increases in synaptic acetylcholine levels in rhesus monkeys. Synapse. 2014;68:556–64.
Sandberg A, Sköld CM, Grunewald J, Eklund A, Wheelock ÅM. Assessing recent smoking status by measuring exhaled carbon monoxide levels. PLoS One. 2011;6:e28864–e28864.
Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The fagerstrom test for nicotine dependence: a revision of the fagerstrom tolerance questionnaire. Br J Addict. 1991;86:1119–27.
Fagerström K. Determinants of tobacco use and renaming the FTND to the fagerström test for cigarette dependence. Nicotine Tob Res. 2012;14:75–78.
Fiore MC, Wetter DW, Bailey WC, Bennett G, Cohen SJ, Dorfman SF, et al. The agency for health care policy and research smoking cessation clinical practice guideline. JAMA 1996;275:1270–80.
Computerized Cognitive Assessment. Clinical Trials: CogState Ltd.
Bois F, Gallezot JD, Zheng MQ, Lin SF, Esterlis I, Cosgrove KP, et al. Evaluation of [(18)F]-(-)-norchlorofluorohomoepibatidine ([(18)F]-(-)-NCFHEB) as a PET radioligand to image the nicotinic acetylcholine receptors in non-human primates. Nucl Med Biol. 2015;42:570–7.
Carson RE, Barker WC, Jeih-San L, Johnson CA. Design of a motion-compensation OSEM list-mode algorithm for resolution-recovery reconstruction for the HRRT. 2003 IEEE Nucl Sci Symp Conf Rec (IEEE Cat No03CH37515). 2003;3285:3281–5.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89.
Lassen NA. Neuroreceptor quantitation in vivo by the steady-state principle using constant infusion or bolus injection of radioactive tracers. J Cereb Blood Flow Metab. 1992;12:709–16.
Carson RE, Channing MA, Blasberg RG, Dunn BB, Cohen RM, Rice KC, et al. Comparison of bolus and infusion methods for receptor quantitation: application to [18F]cyclofoxy and positron emission tomography. J Cereb Blood Flow Metab. 1993;13:24–42.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–9.
Hillmer AT, Carson RE. Quantification of PET infusion studies without true equilibrium: A tissue clearance correction. J Cereb Blood Flow Metab. 2019;40:860–74.
Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, et al. Cigarette smoking saturates brain α4β2 nicotinic acetylcholine receptors. JAMA Psychiatry. 2006;63:907–14.
Bhatt S, Hillmer AT, Nabulsi N, Matuskey D, Lim K, Lin S-F, et al. Evaluation of (-)-[18F]Flubatine-specific binding: Implications for reference region approaches. Synapse. 2018;72:e22016.
Sabri O, Becker G-A, Meyer PM, Hesse S, Wilke S, Graef S, et al. First-in-human PET quantification study of cerebral α4β2* nicotinic acetylcholine receptors using the novel specific radioligand (−)-[18F]Flubatine. Neuroimage. 2015;118:199–208.
Shotbolt P, Tziortzi AC, Searle GE, Colasanti A, van der Aart J, Abanades S, et al. Within-subject comparison of [11C]-(+)-PHNO and [11C]raclopride sensitivity to acute amphetamine challenge in healthy humans. J Cereb Blood Flow Metab 2011;32:127–36.
Narendran R, Frankle WG, Mason NS, Rabiner EA, Gunn RN, Searle GE, et al. Positron emission tomography imaging of amphetamine-induced dopamine release in the human cortex: A comparative evaluation of the high affinity dopamine D2/3 radiotracers [11C]FLB 457 and [11C]fallypride. Synapse. 2009;63:447–61.
Smart K, Naganawa M, Baldassarri SR, Nabulsi N, Ropchan J, Najafzadeh S, et al. PET imaging estimates of regional acetylcholine concentration variation in living human brain. Cereb Cortex. 2021;31:2787–98.
Naganawa M, Nabulsi N, Henry S, Matuskey D, Lin S-F, Slieker L, et al. First-in-human assessment of (11)C-LSN3172176, an M1 muscarinic acetylcholine receptor PET radiotracer. J Nucl Med. 2021;62:553–60.
Hughes JR, Gulliver SB, Fenwick JW, Valliere WA, Cruser K, Pepper S, et al. Smoking cessation among self-quitters. Health Psychol. 1992;11:331–4.
Petrides M, Alivisatos B, Meyer E, Evans AC. Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proc Natl Acad Sci USA. 1993;90:878.
Lara AH, Wallis JD. The role of prefrontal cortex in working memory: a mini review. Front Syst Neurosci. 2015;9:173.
Levin ED. Complex relationships of nicotinic receptor actions and cognitive functions. Biochem Pharm. 2013;86:1145–52.
Koukouli F, Changeux J-P. Do nicotinic receptors modulate high-order cognitive processing? Trends Neurosci. 2020;43:550–64.
Ashare RL, Ray R, Lerman C, Strasser AA. Cognitive effects of the acetylcholinesterase inhibitor, donepezil, in healthy, non-treatment seeking smokers: a pilot feasibility study. Drug Alcohol Depend. 2012;126:263–7.
Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, et al. Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry. 2009;65:144–9.
Chamberlain SR, Odlaug BL, Schreiber LR, Grant JE. Association between tobacco smoking and cognitive functioning in young adults. Am J Addict. 2012;21Suppl 1:S14–19.
Durazzo TC, Meyerhoff DJ, Nixon SJ. A comprehensive assessment of neurocognition in middle-aged chronic cigarette smokers. Drug Alcohol Depend. 2012;122:105–11.
Piper ME, Schlam TR, Cook JW, Sheffer MA, Smith SS, Loh W-Y, et al. Tobacco withdrawal components and their relations with cessation success. Psychopharmacol (Berl). 2011;216:569–78.
Bohnen NI, Grothe MJ, Ray NJ, Müller MLTM, Teipel SJ. Recent advances in cholinergic imaging and cognitive decline—revisiting the cholinergic hypothesis of dementia. Curr Geriatr Rep. 2018;7:1–11.
Acknowledgements
We thank the Yale PET Centre and research assistants Elizabeth Yanac and Ryan Cool for scan management and MRI analysis. We thank Dr. Nii Addy, Dr. Richard Carson, and Dr. Stephanie O’Malley for their insightful scientific contributions.
Funding
This work was supported by the National Institute on Drug Abuse (Grants R01 DA038832, K02 DA031750) and the National Institute on Alcohol Abuse and Alcoholism (Grant K01 AA024788). KCC and SRB were also supported by the National Institute of Neurological Disorders and Stroke (Grant T32 NS041228) and the National Institute on Drug Abuse (Grant K23 DA045957), respectively.
Author information
Authors and Affiliations
Contributions
KPC designed the study. KCC, ATH, JA, BL, SRB, GAA, DM, and KPC supervised recruitment and participation of human volunteers. SRB, GAA, and DM were study physicians. MK, MZ, and YH were study radiochemists. ATH performed image analysis. KCC analyzed clinical data and performed statistical analyses of imaging and clinical data. KCC and KPC drafted the initial manuscript. All authors contributed to editing this article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Calakos, K.C., Hillmer, A.T., Anderson, J.M. et al. Cholinergic system adaptations are associated with cognitive function in people recently abstinent from smoking: a (-)-[18F]flubatine PET study. Neuropsychopharmacol. 48, 683–689 (2023). https://doi.org/10.1038/s41386-023-01535-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-023-01535-1
This article is cited by
-
Multiple sources of β2*-nicotinic acetylcholine receptor binding are differentially affected during tobacco smoking abstinence as revealed by Independent Component Analysis of [18F]Flubatine PET images
Neuropsychopharmacology (2026)
-
Cholinergic tone abnormalities and relationships with smoking severity in human cigarette smokers: exploratory positron emission tomography study using [18F]VAT
Molecular Psychiatry (2025)
-
Integrative biochemical and computational evaluation of methyl eugenol, naphthalene, and linalool as natural multitarget therapeutics for smoking cessation-related metabolic disturbances
Naunyn-Schmiedeberg's Archives of Pharmacology (2025)