Abstract
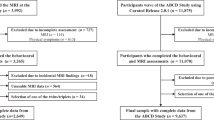
Externalizing psychopathology in childhood is a predictor of poor outcomes across the lifespan. Children exhibiting elevated externalizing symptoms also commonly show emotion dysregulation and callous-unemotional (CU) traits. Examining cross-sectional and longitudinal neural correlates across dimensions linked to externalizing psychopathology during childhood may clarify shared or distinct neurobiological vulnerability for psychopathological impairment later in life. We used tabulated brain structure and behavioural data from baseline, year 1, and year 2 timepoints of the Adolescent Brain Cognitive Development Study (ABCD; baseline n = 10,534). We fit separate linear mixed effect models to examine whether baseline brain structures in frontolimbic and striatal regions (cortical thickness or subcortical volume) were associated with externalizing symptoms, emotion dysregulation, and/or CU traits at baseline and over a two-year period. The most robust relationships found at the cross-sectional level was between cortical thickness in the right rostral middle frontal gyrus and bilateral pars orbitalis was positively associated with CU traits (β = |0.027–0.033|, pcorrected = 0.009–0.03). Over the two-year follow-up period, higher baseline cortical thickness in the left pars triangularis and rostral middle frontal gyrus predicted greater decreases in externalizing symptoms ((F = 6.33–6.94, pcorrected = 0.014). The results of the current study suggest that unique regions within frontolimbic and striatal networks may be more strongly associated with different dimensions of externalizing psychopathology. The longitudinal findings indicate that brain structure in early childhood may provide insight into structural features that influence behaviour over time.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Data for the ABCD Study are available through the National Institutes of Health Data Archive (NDA; nih.nda.gov). The participant IDs included in these analyses and details on the measures used can be found in this project’s NDA study (https://doi.org/10.15154/q36k-ga33).
Code availability
The code for the analysis can be found on GitHub (https://github.com/hajernakua/ext_psychopathology_ABCD).
References
Copeland WE, Shanahan L, Costello J, Angold A. Childhood and adolescent psychiatric disorders as predictors of young adult disorders. Arch Gen Psychiatry. 2009;66:764–72.
Frick PJ, Nigg JT. Current issues in the diagnosis of attention deficit hyperactivity disorder, oppositional defiant disorder, and conduct disorder. Annu Rev Clin Psychol. 2012;8:77–107.
Hansen AS, Christoffersen CH, Telléus GK, Lauritsen MB. Referral patterns to outpatient child and adolescent mental health services and factors associated with referrals being rejected. A cross-sectional observational study. BMC Health Serv Res. 2021;21:1–12.
Wesselhoeft R, Stringaris A, Sibbersen C, Kristensen RV, Bojesen AB, Talati A. Dimensions and subtypes of oppositionality and their relation to comorbidity and psychosocial characteristics. Eur Child Adolesc Psychiatry. 2019;28:351–65.
Bolhuis K, Lubke GH, van der Ende J, Bartels M, van Beijsterveldt CEM, Lichtenstein P, et al. Disentangling Heterogeneity of Childhood Disruptive Behavior Problems Into Dimensions and Subgroups. J Am Acad Child Adolesc Psychiatry. 2017;56:678–86 https://doi.org/10.1016/j.jaac.2017.05.019.
Viding E, McCrory E. Disruptive behavior disorders: the challenge of delineating mechanisms in the face of heterogeneity. Am J Psychiatry. 2020;177:811–7.
Peter F, Andrea S, Nancy A. Forty years of structural brain imaging in mental disorders: is it clinically useful or not? Dialogues Clin Neurosci. 2018;20:179–86.
Brotman MA, Kircanski K, Leibenluft E. Irritability in children and adolescents. Annu Rev Clin Psychol. 2017;13:317–41.
Propp L, Bedard A-CV, Andrade BF Psychopathological and neuropsychological endophenotypes of children with disruptive behaviour. J Behav Cognit Therapy. https://doi.org/10.1016/j.jbct.2020.03.014 (2020).
Kaczkurkin AN, Park SS, Sotiras A, Moore TM, Calkins ME, Cieslak M, et al. Evidence for dissociable linkage of dimensions of psychopathology to brain structure in youths. Am J Psychiatry. 2019;176:1000–9.
Frick PJ, Viding E. Antisocial behavior from a developmental psychopathology perspective. Dev Psychopathol. 2009;21:1111–31.
Herpers PCM, Scheepers FE, Bons DMA, Buitelaar JK, Rommelse NNJ. The cognitive and neural correlates of psychopathy and especially callous-unemotional traits in youths: a systematic review of the evidence. Dev Psychopathol. 2014;26:245–73.
Craig SG, Moretti MM. Profiles of primary and secondary callous-unemotional features in youth: The role of emotion regulation. Dev Psychopathol. 2019;31:1489–500.
Frick PJ, Morris AS. Temperament and developmental pathways to conduct problems. J Clin Child Adolesc Psychol. 2004;33:54–68.
Viding E, Fontaine NMG, McCrory EJ. Antisocial behaviour in children with and without callous-unemotional traits. J R Soc Med. 2012;105:195–200.
Squillaci M, Benoit V. Role of Callous and Unemotional (CU) traits on the development of youth with behavioral disorders: a systematic review. Int J Environ Res Public Health. 2021;18:4712
Michelini G, Palumbo IM, DeYoung CG, Latzman RD, Kotov R. Linking RDoC and HiTOP: A new interface for advancing psychiatric nosology and neuroscience. Clin Psychol Rev. 2021;86:102025.
Graziano PA, Landis T, Maharaj A, Ros-Demarize R, Hart KC, Garcia A. Differentiating preschool children with conduct problems and callous-unemotional behaviors through emotion regulation and executive functioning. J Clin Child Adolesc Psychol. 2022;51:170–82.
Northam JC, Dadds MR. Is Callous Always Cold? A critical review of the literature on emotion and the development of callous-unemotional traits in children. Clin Child Fam Psychol Rev. 2020;23:265–83.
De Brito, Mechelli A, Wilke M, Laurens KR, Jones AP, Barker GJ, et al. Size matters: increased grey matter in boys with conduct problems and callous-unemotional traits. Brain. 2009;132:843–52.
Mulraney M, Sciberras E, Gulenc A, Efron D, Hazell P, Silk TJ. Neural correlates of irritability in a community sample of children. J Affect Disord. 2021;292:223–6.
Chaarani B, Kan K-J, Mackey S, Spechler PA, Potter A, Banaschewski T, et al. Neural correlates of adolescent irritability and its comorbidity with psychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2020;59:1371–9.
Fairchild G, Hagan CC, Walsh ND, Passamonti L, Calder AJ, Goodyer IM. Brain structure abnormalities in adolescent girls with conduct disorder. J Child Psychol Psychiatry. 2013;54:86–95.
Seymour KE, Tang X, Crocetti D, Mostofsky SH, Miller MI, Rosch KS. Anomalous subcortical morphology in boys, but not girls, with ADHD compared to typically developing controls and correlates with emotion dysregulation. Psychiatry Research: Neuroimaging. 2017;261:20–8.
Waller R, Hawes SW, Byrd AL, Dick AS, Sutherland MT, Riedel MC, et al. Disruptive behavior problems, callous-unemotional traits, and regional gray matter volume in the adolescent brain and cognitive development study. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:481–9.
Tsai C-J, Lin H-Y, Tseng IW-Y, Gau SS-F Brain voxel-based morphometry correlates of emotion dysregulation in attention-deficit hyperactivity disorder. Brain Imaging Behav. 2020;15:1338–02
Cha J, Fekete T, Siciliano F, Biezonski D, Greenhill L, Pliszka SR, et al. Neural correlates of aggression in medication-naive children with ADHD: Multivariate analysis of morphometry and tractography. Neuropsychopharmacology. 2015;40:1717–25
Vijayakumar N, Whittle S, Yücel M, Dennison M, Simmons J, Allen NB. Thinning of the lateral prefrontal cortex during adolescence predicts emotion regulation in females. Soc Cogn Affect Neurosci. 2014;9:1845–54.
Lyall AE, Shi F, Geng X, Woolson S, Li G, Wang L, et al. Dynamic development of regional cortical thickness and surface area in early childhood. Cereb Cortex. 2015;25:2204–12.
Norbom LB, Ferschmann L, Parker N, Agartz I, Andreassen OA, Paus T, et al. New insights into the dynamic development of the cerebral cortex in childhood and adolescence: Integrating macro- and microstructural MRI findings. Prog Neurobiol. 2021;204:102109.
Barch DM, Albaugh MD, Avenevoli S, Chang L, Clark DB, Glantz MD, et al. Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: Rationale and description. Dev Cogn Neurosci. 2018;32:55–66.
Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, et al. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci. 2018;32:43–54.
Hagler DJ Jr, Hatton S, Cornejo MD, Makowski C, Fair DA, et al. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage. 2019;202:116091.
Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94.
Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–5.
Ameis SH, Ducharme S, Albaugh MD, Hudziak JJ, Botteron KN, Lepage C, et al. Cortical thickness, cortico-amygdalar networks, and externalizing behaviors in healthy children. Biol Psychiatry. 2014;75:65–72.
Albaugh MD, Ducharme S, Karama S, Watts R, Lewis JD, Orr C, et al. Anxious/depressed symptoms are related to microstructural maturation of white matter in typically developing youths. Dev Psychopathol. 2017;29:751–8.
Ducharme S, Albaugh MD, Nguyen T-V, Hudziak JJ, Mateos-Pérez JM, Labbe A, et al. Trajectories of cortical thickness maturation in normal brain development-The importance of quality control procedures. Neuroimage. 2016;125:267–79.
Nakua H, Yu J-C, Abdi H, Hawco C, Voineskos A, Hill S, et al. Comparing the stability and reproducibility of brain-behavior relationships found using canonical correlation analysis and partial least squares within the ABCD sample. Netw Neurosci. 2024;8:576–96.
Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, et al. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;603:654–60.
Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; 2013.
Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8.
Townsend L, Kobak K, Kearney C, Milham M, Andreotti C, Escalera J, et al. Development of three web-based computerized versions of the kiddie schedule for affective disorders and schizophrenia child psychiatric diagnostic interview: preliminary validity data. J Am Acad Child Adolesc Psychiatry. 2020;59:309–25.
Karcher NR, Loewy RL, Savill M, Avenevoli S, Huber RS, Simon TJ, et al. Replication of associations with psychotic-like experiences in middle childhood from the adolescent brain cognitive development (ABCD) study. Schizophr Bull Open. 2020;1:sgaa009.
Karcher NR, Niendam TA, Barch DM. Adverse childhood experiences and psychotic-like experiences are associated above and beyond shared correlates: Findings from the adolescent brain cognitive development study. Schizophr Res. 2020;222:235–42.
Achenbach TM Manual for the ASEBA School-age Forms & Profiles: An Integrated System of Multi-informant Assessment. ASEBA; 2001.
Aitken M, Battaglia M, Marino C, Mahendran N, Andrade BF. Clinical utility of the CBCL Dysregulation Profile in children with disruptive behavior. J Affect Disord. 2019;253:87–95.
Masi G, Muratori P, Manfredi A, Pisano S, Milone A. Child behaviour checklist emotional dysregulation profiles in youth with disruptive behaviour disorders: clinical correlates and treatment implications. Psychiatry Res. 2015;225:191–6.
Hawes SW, Waller R, Byrd AL, Bjork JM, Dick AS, Sutherland MT, et al. Reward processing in children with disruptive behavior disorders and callous-unemotional traits in the ABCD study. Am J Psychiatry. 2021;178:333–42.
Hawes SW, Waller R, Thompson WK, Hyde LW, Byrd AL, Burt SA, et al. Assessing callous-unemotional traits: development of a brief, reliable measure in a large and diverse sample of preadolescent youth. Psychol Med. 2020;50:456–64.
Goodman R, Ford T, Richards H, Gatward R, Meltzer H. The Development and Well-Being Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry. 2000;41:645–55.
Lumley T, Diehr P, Emerson S, Chen L. The importance of the normality assumption in large public health data sets. Annu Rev Public Health. 2002;23:151–69.
Rakesh D, Cropley V, Zalesky A, Vijayakumar N, Allen NB, Whittle S. Neighborhood disadvantage and longitudinal brain-predicted-age trajectory during adolescence. Dev Cogn Neurosci. 2021;51:101002.
Hill WD, Hagenaars SP, Marioni RE, Harris SE, Liewald DCM, Davies G, et al. Molecular genetic contributions to social deprivation and household income in UK Biobank. Curr Biol. 2016;26:3083–9.
Bates D, Mächler M, Bolker B, Walker S Fitting Linear Mixed-Effects Models using lme4. ArXiv [StatCO]. 2014.
R Core Team. R: A language and environment for statistical computing. 2021.
Zhao Y, Paulus M, Bagot KS, Constable RT, Yaggi HK, Redeker NS, et al. Brain structural covariation linked to screen media activity and externalizing behaviors in children. J Behav Addict. 2022;11:417–26.
Wu X, Palaniyappan L, Yu G, Zhang K, Seidlitz J, Liu Z, et al. Morphometric dis-similarity between cortical and subcortical areas underlies cognitive function and psychiatric symptomatology: a preadolescence study from ABCD. Mol Psychiatry. https://doi.org/10.1038/s41380-022-01896-x (2022).
Damme KSF, Norton ES, Briggs-Gowan MJ, Wakschlag LS, Mittal VA. Developmental patterning of irritability enhances prediction of psychopathology in preadolescence: Improving RDoC with developmental science. J Psychopathol Clin Sci. 2022;131:556–66.
Seok J-W, Bajaj S, Soltis-Vaughan B, Lerdahl A, Garvey W, Bohn A, et al. Structural atrophy of the right superior frontal gyrus in adolescents with severe irritability. Hum Brain Mapp. 2021;42:4611–22.
Luna B, Marek S, Larsen B, Tervo-Clemmens B, Chahal R. An integrative model of the maturation of cognitive control. Annu Rev Neurosci. 2015;38:151–70.
Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–9.
Fairchild G, Hawes DJ, Frick PJ Conduct disorder. Nat Rev. 2019. 2019.
De Brito, Forth AE, Baskin-Sommers AR, Brazil IA, Kimonis ER, Pardini D, et al. Psychopathy. Nat Rev Dis Primers. 2021;7:49.
Caldwell BM, Anderson NE, Harenski KA, Sitney MH, Caldwell MF, Van Rybroek GJ, et al. The structural brain correlates of callous-unemotional traits in incarcerated male adolescents. Neuroimage Clin. 2019;22:101703.
Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, et al. How does your cortex grow? J Neurosci. 2011;31:7174–7.
Gilmore JH, Knickmeyer RC, Gao W. Imaging structural and functional brain development in early childhood. Nat Rev Neurosci. 2018;19:123–37.
Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, et al. Puberty-related influences on brain development. Mol Cell Endocrinol. 2006;254–255:154–62.
Parker N, Patel Y, Jackowski AP, Pan PM, Salum GA, Pausova Z, et al. Assessment of neurobiological mechanisms of cortical thinning during childhood and adolescence and their implications for psychiatric disorders. JAMA Psychiatry. 2020;77:1127–36.
Tanzer M, Derome M, Morosan L, Salaminios G, Debbané M. Cortical thickness of the insula and prefrontal cortex relates to externalizing behavior: Cross-sectional and prospective findings. Dev Psychopathol. 2021;33:1437–47.
Segal A, Parkes L, Aquino K, Kia SM, Wolfers T, Franke B, et al. Regional, circuit and network heterogeneity of brain abnormalities in psychiatric disorders. Nat Neurosci. 2023;26:1613–29.
Cardenas-Iniguez C, Gonzalez MR “We controlled for race and ethnicity…” Recommendations for the responsible use and communication of race and ethnicity in neuroimaging research. PsyArXiv. 2023.
Chad-Friedman E, Galano MM, Lemay EP, Olino TM, Klein DN, Dougherty LR. Parsing between- and within-person effects: Longitudinal associations between irritability and internalizing and externalizing problems from early childhood through adolescence. Dev Psychopathol. 2021;35:1–11.
Tooley UA, Bassett DS, Mackey AP. Environmental influences on the pace of brain development. Nat Rev Neurosci. 2021;22:372–84.
Kretschmer T, Vrijen C, Nolte IM, Wertz J, Hartman CA. Gene-environment interplay in externalizing behavior from childhood through adulthood. J Child Psychol Psychiatry. 2022;63:1206–13.
Herd T, Jacques K, Brieant A, Noll JG, King-Casas B, Kim-Spoon J Parenting, emotion regulation, and externalizing symptomatology as adolescent antecedents to young adult health risk behaviors. J Res Adolesc. https://doi.org/10.1111/jora.12831 (2023).
Acknowledgements
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development® (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9–10 and follow them over 10 years into early adulthood. The ABCD Study® is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortiummembers/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from NDA Release 4.0 (https://doi.org/10.15154/q36k-ga33).
Funding
LP has received funding from a Canadian Institutes of Health Research (CIHR) Doctoral Award and Ontario Graduate Scholarship. HN has received funding from the CAMH Discovery Fund, Ontario Graduate Scholarship, Fulbright Canada, and currently receives funding from the CIHR Doctoral Award. SHA currently receives funding from the NIMH, CIHR, the CAMH Foundation, and the Canada Research Chairs Program. BA currently receives funds from the CIHR, CAMH Discovery Fund, LesLois Shaw Foundation and Peter Gilgan Foundation.
Author information
Authors and Affiliations
Contributions
LP and HN conducted all the analyses and wrote the main draft of the paper. BA, ACB, and SA contributed to the design of the study. MS contributed to the design of the analytical approaches used. All authors wrote, revised, approved, and agreed to be accountable for all aspects of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nakua, H., Propp, L., Bedard, AC.V. et al. Investigating cross-sectional and longitudinal relationships between brain structure and distinct dimensions of externalizing psychopathology in the ABCD sample. Neuropsychopharmacol. 50, 499–506 (2025). https://doi.org/10.1038/s41386-024-02000-3
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41386-024-02000-3