Abstract
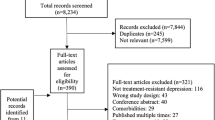
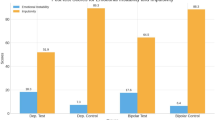
In response to restrictions on electroconvulsive therapy (ECT) access during COVID-19, we designed a trial to assess the clinical outcomes service impacts, employing an extended course of accelerated intermittent theta burst stimulation (aiTBS), in patients with moderate to severe depression in need of ECT. This open label clinical trial was comprised of 3 phases: (i) an acute phase, where iTBS treatments were administered 8 times daily, for up to 10 days; (ii) a tapering phase of 2 treatment days per week for 2 weeks, followed by 1 treatment day per week for 2 weeks; and (iii) a symptom-based relapse prevention phase, whereby treatments were scheduled based on symptom re-emergence, for up to 6 months. Of the 155 patients who completed the acute phase of the study, the remission rate was 16.1%. The mean reduction from baseline on the HRSD-24 was 29.4% (p < 0.001) and the response rate was 25.2%. Of the 110 patients who completed the tapering phase, the mean reduction from baseline was 42.6% (p < 0.001) and response and remission rates were 49.6% and 34.8%, respectively. Of the 61 patients who were eligible for the relapse prevention phase, 43 completed, with a mean reduction from baseline of 60.1% (p < 0.001); 7 patients relapsed during this phase. This study demonstrated that an extended aiTBS protocol safely led to meaningful clinical outcomes in patients with severe depression, who otherwise would have received ECT, and thus reduced pressure on ECT services during the pandemic.
Trial Registration
ClinicalTrials.gov Identifier: NCT04384965 (https://clinicaltrials.gov/study/NCT04384965?term=NCT04384965&rank=1)
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Spaans HP, Verwijk E, Comijs HC, Kok RM, Sienaert P, Bouckaert F, et al. Efficacy and cognitive side effects after brief pulse and ultrabrief pulse right unilateral electroconvulsive therapy for major depression: a randomized, double-blind, controlled study. J Clin Psychiatry. 2013;74:e1029–36. https://doi.org/10.4088/JCP.13m08538.
Angus H, Williams D, Anderson M Memorandum to Ontario Health and hospitals. Ramping down elective surgeries and other non-emergent activities. Toronto: Ministry of Health; 2020.
Williams DC Directive #2 for health care providers (regulated health professionals or persons who operate a group practice of regulated health professionals) 2020.
Fink M, Kellner CH, McCall WV. The role of ECT in suicide prevention. J ECT. 2014;30:5–9. https://doi.org/10.1097/YCT.0b013e3182a6ad0d.
Kaster TS, Blumberger DM, Gomes T, Sutradhar R, Wijeysundera DN, Vigod SN. Risk of suicide death following electroconvulsive therapy treatment for depression: a propensity score-weighted, retrospective cohort study in Canada. Lancet Psychiatry. 2022;9:435–46. https://doi.org/10.1016/S2215-0366(22)00077-3.
Demchenko I, Blumberger DM, Flint AJ, Anderson M, Daskalakis ZJ, Foley K, et al. Electroconvulsive therapy in Canada during the first wave of COVID-19: results of the “What Happened” national survey. J ECT. 2022;38:52–9. https://doi.org/10.1097/YCT.0000000000000801.
Robertson J, Flint AJ, Blumberger D, Bhat V. Ethical considerations in providing electroconvulsive therapy during the COVID-19 pandemic. Can J Psychiatry. 2021;66:701–6. https://doi.org/10.1177/0706743721993617.
Blumberger DM, Vila-Rodriguez F, Thorpe KE, Feffer K, Noda Y, Giacobbe P, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. 2018;391:1683–92. https://doi.org/10.1016/S0140-6736(18)30295-2.
Di Lazzaro V, Dileone M, Pilato F, Capone F, Musumeci G, Ranieri F, et al. Modulation of motor cortex neuronal networks by rTMS: comparison of local and remote effects of six different protocols of stimulation. J Neurophysiol. 2011;105:2150–6. https://doi.org/10.1152/jn.00781.2010.
Kaster TS, Downar J, Vila-Rodriguez F, Thorpe KE, Feffer K, Noda Y, et al. Trajectories of response to dorsolateral prefrontal rTMS in major depression: a THREE-D study. Am J Psychiatry. 2019;176:367–75. https://doi.org/10.1176/appi.ajp.2018.18091096.
Trevizol AP, Downar J, Vila-Rodriguez F, Thorpe KE, Daskalakis ZJ, Blumberger DM. Predictors of remission after repetitive transcranial magnetic stimulation for the treatment of major depressive disorder: An analysis from the randomised non-inferiority THREE-D trial. EClinicalMedicine. 2020;22:100349 https://doi.org/10.1016/j.eclinm.2020.100349.
Chen JJ, Zhao LB, Liu YY, Fan SH, Xie P. Comparative efficacy and acceptability of electroconvulsive therapy versus repetitive transcranial magnetic stimulation for major depression: A systematic review and multiple-treatments meta-analysis. Behav Brain Res. 2017;320:30–6. https://doi.org/10.1016/j.bbr.2016.11.028.
Minichino A, Bersani FS, Capra E, Pannese R, Bonanno C, Salviati M, et al. ECT, rTMS, and deepTMS in pharmacoresistant drug-free patients with unipolar depression: a comparative review. Neuropsychiatr Dis Treat. 2012;8:55–64. https://doi.org/10.2147/NDT.S27025.
Chen L, Klooster DCW, Tik M, Thomas EHX, Downar J, Fitzgerald PB, et al. Accelerated repetitive transcranial magnetic stimulation to treat major depression: the past, present, and future. Harv Rev Psychiatry. 2023;31:142–61. https://doi.org/10.1097/HRP.0000000000000364.
Williams NR, Sudheimer KD, Bentzley BS, Pannu J, Stimpson KH, Duvio D, et al. High-dose spaced theta-burst TMS as a rapid-acting antidepressant in highly refractory depression. Brain. 2018;141:e18 https://doi.org/10.1093/brain/awx379.
Cole EJ, Phillips AL, Bentzley BS, Stimpson KH, Nejad R, Barmak F, et al. Stanford neuromodulation therapy (SNT): a double-blind randomized controlled trial. Am J Psychiatry. 2022;179:132–41. https://doi.org/10.1176/appi.ajp.2021.20101429.
Cole EJ, Stimpson KH, Bentzley BS, Gulser M, Cherian K, Tischler C, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry. 2020;177:716–26. https://doi.org/10.1176/appi.ajp.2019.19070720.
Sackeim HA, Haskett RF, Mulsant BH, Thase ME, Mann JJ, Pettinati HM, et al. Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: a randomized controlled trial. JAMA. 2001;285:1299–307. https://doi.org/10.1001/jama.285.10.1299.
Geoly AD, Kratter IH, Toosi P, Cole EJ, Sahlem GL, Williams NR. Sustained efficacy of stanford neuromodulation therapy (SNT) in open-label repeated treatment. Am J Psychiatry. 2024;181:71–3. https://doi.org/10.1176/appi.ajp.20230113.
Lisanby SH, Sampson S, Husain MM, Petrides G, Knapp RG, McCall WV, et al. Toward individualized post-electroconvulsive therapy care: piloting the Symptom-Titrated, Algorithm-Based Longitudinal ECT (STABLE) intervention. J ECT. 2008;24:179–82. https://doi.org/10.1097/YCT.0b013e318185fa6b.
Lisanby SH, McClintock SM, McCall WV, Knapp RG, Cullum CM, Mueller M, et al. Longitudinal neurocognitive effects of combined electroconvulsive therapy (ECT) and pharmacotherapy in major depressive disorder in older adults: phase 2 of the PRIDE study. Am J Geriatr Psychiatry. 2022;30:15–28. https://doi.org/10.1016/j.jagp.2021.04.006.
Blumberger DM, Daskalakis ZJ, Vila-Rodriguez F, Boivin-Lafleur D, Goodman MS, Kaster TS, et al. Accelerated intermittent theta burst as a substitute for patients needing electroconvulsive therapy during the COVID-19 pandemic: study protocol for an open-label clinical trial. medRxiv. 2020. https://doi.org/10.1101/2020.12.15.20248260.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33.
Mir-Moghtadaei A, Caballero R, Fried P, Fox MD, Lee K, Giacobbe P, et al. Concordance between BeamF3 and MRI-neuronavigated target sites for repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex. Brain Stimul. 2015;8:965–73. https://doi.org/10.1016/j.brs.2015.05.008.
Konstantinou GN, Downar J, Daskalakis ZJ, Blumberger DM. Accelerated intermittent theta burst stimulation in late-life depression: a possible option for older depressed adults in need of ECT during the COVID-19 pandemic. Am J Geriatr Psychiatry. 2020;28:1025–9. https://doi.org/10.1016/j.jagp.2020.07.007.
Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–6. https://doi.org/10.1016/j.neuron.2004.12.033.
Desforges M, Hadas I, Mihov B, Morin Y, Rochette Braun M, Lioumis P, et al. Dose-response of intermittent theta burst stimulation of the prefrontal cortex: A TMS-EEG study. Clin Neurophysiol. 2022;136:158–72. https://doi.org/10.1016/j.clinph.2021.12.018.
Richard M, Noiseux C, Desbeaumes Jodoin V, Blumberger DM, Sheen J, Mansouri F, et al. Prolonged intermittent theta burst stimulation in the treatment of major depressive disorder: a case series. Psychiatry Res. 2022;315:114709 https://doi.org/10.1016/j.psychres.2022.114709.
Li CT, Cheng CM, Lin HC, Yeh SH, Jeng JS, Wu HT, et al. The longer, the better ? Longer left-sided prolonged intermittent theta burst stimulation in patients with major depressive disorder: A randomized sham-controlled study. Asian J Psychiatr. 2023;87:103686 https://doi.org/10.1016/j.ajp.2023.103686.
Gamboa OL, Antal A, Moliadze V, Paulus W. Simply longer is not better: reversal of theta burst after-effect with prolonged stimulation. Exp Brain Res. 2010;204:181–7. https://doi.org/10.1007/s00221-010-2293-4.
Kramar EA, Babayan AH, Gavin CF, Cox CD, Jafari M, Gall CM, et al. Synaptic evidence for the efficacy of spaced learning. Proc Natl Acad Sci USA. 2012;109:5121–6. https://doi.org/10.1073/pnas.1120700109.
Lynch G, Kramar EA, Babayan AH, Rumbaugh G, Gall CM. Differences between synaptic plasticity thresholds result in new timing rules for maximizing long-term potentiation. Neuropharmacology. 2013;64:27–36. https://doi.org/10.1016/j.neuropharm.2012.07.006.
Smolen P, Zhang Y, Byrne JH. The right time to learn: mechanisms and optimization of spaced learning. Nat Rev Neurosci. 2016;17:77–88. https://doi.org/10.1038/nrn.2015.18.
Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–96. https://doi.org/10.1111/j.2044-8260.1967.tb00530.x.
Hausmann A, Post T, Post F, Dehning J, Kemmler G, Grunze H. Efficacy of continuation/maintenance electroconvulsive therapy in the treatment of patients with mood disorders: a retrospective analysis. J ECT. 2019;35:122–6. https://doi.org/10.1097/YCT.0000000000000547.
Odeberg H, Rodriguez-Silva B, Salander P, Martensson B. Individualized continuation electroconvulsive therapy and medication as a bridge to relapse prevention after an index course of electroconvulsive therapy in severe mood disorders: a naturalistic 3-year cohort study. J ECT. 2008;24:183–90. https://doi.org/10.1097/YCT.0b013e318177275d.
Brakemeier EL, Merkl A, Wilbertz G, Quante A, Regen F, Buhrsch N, et al. Cognitive-behavioral therapy as continuation treatment to sustain response after electroconvulsive therapy in depression: a randomized controlled trial. Biol Psychiatry. 2014;76:194–202. https://doi.org/10.1016/j.biopsych.2013.11.030.
Kellner CH, Knapp RG, Petrides G, Rummans TA, Husain MM, Rasmussen K, et al. Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression: a multisite study from the Consortium for Research in Electroconvulsive Therapy (CORE). Arch Gen Psychiatry. 2006;63:1337–44. https://doi.org/10.1001/archpsyc.63.12.1337.
Beck AT, Steer RA, Brown GK Beck Depression Inventory-Second Edition Manual. San Antonio, TX: The Psychological Corporation; 1996.
Kellner CH, Husain MM, Knapp RG, McCall WV, Petrides G, Rudorfer MV, et al. Right unilateral ultrabrief pulse ECT in geriatric depression: phase 1 of the PRIDE study. Am J Psychiatry. 2016;173:1101–9. https://doi.org/10.1176/appi.ajp.2016.15081101.
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. https://doi.org/10.1046/j.1525-1497.2001.016009606.x.
Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–7. https://doi.org/10.1001/archinte.166.10.1092.
Ustun TB, Chatterji S, Kostanjsek N, Rehm J, Kennedy C, Epping-Jordan J, et al. Developing the World Health Organization Disability Assessment Schedule 2.0. Bull World Health Organ. 2010;88:815–23. https://doi.org/10.2471/BLT.09.067231.
Dunner DL, Aaronson ST, Sackeim HA, Janicak PG, Carpenter LL, Boyadjis T, et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a 1-year follow-up period. J Clin Psychiatry. 2014;75:1394–401. https://doi.org/10.4088/JCP.13m08977.
Morrens J, Mathews M, Popova V, Borentain S, Rive B, Gonzalez Martin Moro B, et al. Use of Clinical Global Impressions-Severity (CGI-S) to Assess Response to Antidepressant Treatment in Patients with Treatment-Resistant Depression. Neuropsychiatr Dis Treat. 2022;18:1127–32. https://doi.org/10.2147/NDT.S358367.
Duprat R, Desmyter S, Rudi de R, van Heeringen K, Van den Abbeele D, Tandt H, et al. Accelerated intermittent theta burst stimulation treatment in medication-resistant major depression: A fast road to remission? J Affect Disord. 2016;200:6–14. https://doi.org/10.1016/j.jad.2016.04.015.
McGirr A, Van den Eynde F, Tovar-Perdomo S, Fleck MP, Berlim MT. Effectiveness and acceptability of accelerated repetitive transcranial magnetic stimulation (rTMS) for treatment-resistant major depressive disorder: an open label trial. J Affect Disord. 2015;173:216–20. https://doi.org/10.1016/j.jad.2014.10.068.
Stokes MG, Chambers CD, Gould IC, Henderson TR, Janko NE, Allen NB, et al. Simple metric for scaling motor threshold based on scalp-cortex distance: application to studies using transcranial magnetic stimulation. J Neurophysiol. 2005;94:4520–7. https://doi.org/10.1152/jn.00067.2005.
Deng ZD, Luber B, McClintock SM, Weiner RD, Husain MM, Lisanby SH. Clinical outcomes of magnetic seizure therapy vs electroconvulsive therapy for major depressive episode: a randomized clinical trial. JAMA Psychiatry. 2023. https://doi.org/10.1001/jamapsychiatry.2023.4599.
Blumberger DM, Vila-Rodriguez F, Wang W, Knyahnytska Y, Butterfield M, Noda Y, et al. A randomized sham controlled comparison of once vs twice-daily intermittent theta burst stimulation in depression: A Canadian rTMS treatment and biomarker network in depression (CARTBIND) study. Brain Stimul. 2021;14:1447–55. https://doi.org/10.1016/j.brs.2021.09.003.
Pridmore S, May T. Relapse prevention (RP) TMS. Brain Stimul. 2018;11:1391–2. https://doi.org/10.1016/j.brs.2018.08.004.
Rachid F. Maintenance repetitive transcranial magnetic stimulation (rTMS) for relapse prevention in with depression: a review. Psychiatry Res. 2018;262:363–72. https://doi.org/10.1016/j.psychres.2017.09.009.
Fitzgerald PB, Grace N, Hoy KE, Bailey M, Daskalakis ZJ. An open label trial of clustered maintenance rTMS for patients with refractory depression. Brain Stimul. 2013;6:292–7. https://doi.org/10.1016/j.brs.2012.05.003.
Funding
This work was supported by the Innovation Fund of the Alternative Funding Plan for the Academic Health Sciences Centers of Ontario (grant no. 1000890). We would also like to acknowledge the Temerty Center Foundation and the Center for Addiction and Mental Health Foundation.
Author information
Authors and Affiliations
Contributions
DMB conceived and designed the study. JD, FVR, ZJD, DV, TSK, YK provided input on the study design. APT, GNK, DBL, RB provided medical care or performed motor thresholds for participants. DMB and MSG developed the statistical analysis plan. MSG completed the data analysis. All authors contributed to the interpretation of data. MSG drafted the manuscript. All authors contributed to critical revisions of the manuscript. DMB had final responsibility for submission of the manuscript.
Corresponding author
Ethics declarations
Competing interests
DV holds the Labatt Family Professorship in Depression Biology, a University Named Professorship at the University of Toronto. She receives research support from CIHR, NIMH, the Center for Addiction and Mental Health (CAMH), The Center for Mental Health at University Health Network and the Department of Psychiatry at the University of Toronto. DV declares no biomedical interests or conflicts. FVR receives research support from CIHR, Brain Canada, Michael Smith Foundation for Health Research, Vancouver Coastal Health Research Institute, and Weston Brain Institute for investigator-initiated research. Philanthropic support from Seedlings Foundation. In-kind equipment support for investigator-initiated research from MagVenture. He has received honoraria for participation in an advisory board for Allergan. FVR is a volunteer director on the board of directors of the British Columbia Schizophrenia Society. TSK receives research support from the Canadian Institutes of Health Research, Patient-Centered Outcomes Research Institute, and the AFP Innovation Fund In the last 10 years, ZJD has received research and equipment in-kind support for an investigator-initiated study through Brainsway Inc and Magventure Inc. He also currently serves on the scientific advisory board for Brainsway Inc. His work has been supported by the National Institutes of Mental Health (NIMH), the Canadian Institutes of Health Research (CIHR), Brain Canada and the Temerty Family, Grant and Kreutzkamp Family Foundations. DMB receives research support from CIHR, NIH, Brain Canada and the Temerty Family through the CAMH Foundation and the Campbell Family Research Institute. He received research support and in-kind equipment support for an investigator-initiated study from Brainsway Ltd. He was the site principal investigator for three sponsor-initiated studies for Brainsway Ltd. He also received in-kind equipment support from Magventure for two investigator-initiated studies. He received medication supplies for an investigator-initiated trial from Indivior. He is a scientific advisor for Sooma Medical. He is the Co-Chair of the Clinical Standards Committee of the Clinical TMS Society (unpaid). The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goodman, M.S., Trevizol, A.P., Konstantinou, G.N. et al. Extended course accelerated intermittent theta burst stimulation as a substitute for depressed patients needing electroconvulsive therapy. Neuropsychopharmacol. 50, 685–694 (2025). https://doi.org/10.1038/s41386-024-02007-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-024-02007-w