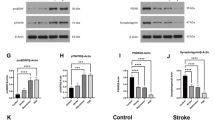
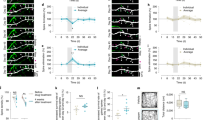
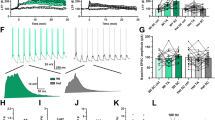
Abstract
Angelman syndrome (AS) is a rare genetic neurodevelopmental disorder with profoundly debilitating symptoms with no FDA-approved cure or therapeutic. Brain-derived neurotrophic factor (BDNF), and its receptor tropomyosin receptor kinase B (TrkB), have a well-established role as regulators of synaptic plasticity, dendritic outgrowth and spine formation. Previously, we reported that the association of postsynaptic density protein 95 (PSD-95) with TrkB is critical for intact BDNF signaling in the AS mouse model, as illustrated by attenuated PLCγ and PI3K signaling and intact MAPK pathway signaling. These data suggest that drugs tailored to enhance the TrkB-PSD-95 interaction may provide a novel approach for the treatment of AS and a variety of neurodevelopmental disorders (NDDs). To evaluate this critical interaction, we synthesized a class of high-affinity PSD-95 ligands that bind specifically to the PDZ3 domain of PSD-95, denoted as Syn3 peptidomimetic ligands. We evaluated Syn3 and its analog D-Syn3 (engineered using dextrorotary (D)-amino acids) in vivo using the Ube3a exon 2 deletion mouse model of AS. Following systemic administration of Syn3 and D-Syn3, we demonstrate improvement in the seizure domain of AS. Learning and memory using the novel object recognition assay also illustrated improved cognition following Syn3 and D-Syn3, along with restored long-term potentiation. A pharmacokinetic analysis of D-Syn3 demonstrates that it crosses the blood-brain barrier (BBB), and the brain influx rate is in the range of CNS therapeutics. Finally, D-Syn3 treated mice showed a partial rescue in motor learning. Neither Syn3 nor D-Syn3 improved gross exploratory locomotion deficits, nor gait impairments that have been well documented in the AS rodent models. These findings highlight the need for further investigation of this compound class as a potential therapeutic for AS and other genetic NDDs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Beaudet AL. Angelman syndrome: Drugs to awaken a paternal gene. Nature. 2011;481:150–2.
Buiting K, Williams C, Horsthemke B. Angelman syndrome - insights into a rare neurogenetic disorder. Nat Rev Neurol. 2016;12:584–93.
Williams CA. Neurological aspects of the Angelman syndrome. Brain Dev. 2005;27:88–94.
Williams CA. The behavioral phenotype of the Angelman syndrome. Am J Med Genet C Semin Med Genet. 2010;154C:432–7.
Petkova SP, Adhikari A, Berg EL, Fenton TA, Duis J, Silverman JL. Gait as a quantitative translational outcome measure in Angelman syndrome. Autism Res. 2022;15:821–33.
Born HA, Dao AT, Levine AT, Lee WL, Mehta NM, Mehra S, et al. Strain-dependence of the Angelman Syndrome phenotypes in Ube3a maternal deficiency mice. Sci Rep. 2017;7:8451.
Adhikari A, Copping NA, Beegle J, Cameron DL, Deng P, O’Geen H, et al. Functional rescue in an Angelman syndrome model following treatment with lentivector transduced hematopoietic stem cells. Hum Mol Genet. 2021;30:1067–83.
Jiang YH, Armstrong D, Albrecht U, Atkins CM, Noebels JL, Eichele G, et al. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron. 1998;21:799–811.
Copping NA, Silverman JL. Abnormal electrophysiological phenotypes and sleep deficits in a mouse model of Angelman Syndrome. Mol Autism. 2021;12:9.
Berg EL, Petkova SP, Born HA, Adhikari A, Anderson AE, Silverman JL. Insulin-like growth factor-2 does not improve behavioral deficits in mouse and rat models of Angelman Syndrome. Mol Autism. 2021;12:59.
Huang HS, Burns AJ, Nonneman RJ, Baker LK, Riddick NV, Nikolova VD, et al. Behavioral deficits in an Angelman syndrome model: effects of genetic background and age. Behav Brain Res. 2013;243:79–90.
Baudry M, Kramar E, Xu X, Zadran H, Moreno S, Lynch G, et al. Ampakines promote spine actin polymerization, long-term potentiation, and learning in a mouse model of Angelman syndrome. Neurobiol Dis. 2012;47:210–5.
Dindot SV, Antalffy BA, Bhattacharjee MB, Beaudet AL. The Angelman syndrome ubiquitin ligase localizes to the synapse and nucleus, and maternal deficiency results in abnormal dendritic spine morphology. Hum Mol Genet. 2008;17:111–8.
Khatri N, Gilbert JP, Huo Y, Sharaflari R, Nee M, Qiao H, et al. The Autism Protein Ube3A/E6AP Remodels Neuronal Dendritic Arborization via Caspase-Dependent Microtubule Destabilization. J Neurosci. 2018;38:363–78.
Kim H, Kunz PA, Mooney R, Philpot BD, Smith SL. Maternal Loss of Ube3a Impairs Experience-Driven Dendritic Spine Maintenance in the Developing Visual Cortex. J Neurosci. 2016;36:4888–94.
Sun J, Liu Y, Tran J, O’Neal P, Baudry M, Bi X. mTORC1-S6K1 inhibition or mTORC2 activation improves hippocampal synaptic plasticity and learning in Angelman syndrome mice. Cell Mol Life Sci. 2016;73:4303–14.
Chao MV. Trophic factors: An evolutionary cul-de-sac or door into higher neuronal function? J Neurosci Res. 2000;59:353–5.
Lauterborn JC, Pineda E, Chen LY, Ramirez EA, Lynch G, Gall CM. Ampakines cause sustained increases in brain-derived neurotrophic factor signaling at excitatory synapses without changes in AMPA receptor subunit expression. Neuroscience. 2009;159:283–95.
Leal G, Afonso PM, Salazar IL, Duarte CB. Regulation of hippocampal synaptic plasticity by BDNF. Brain Res. 2015;1621:82–101.
Li G, Qiu S. Neurodevelopmental Underpinnings of Angelman Syndrome. J Bioanal Biomed. 2014;6:052056.
Lynch G, Rex CS, Chen LY, Gall CM. The substrates of memory: defects, treatments, and enhancement. Eur J Pharmacol. 2008;585:2–13.
Monteggia LM. Toward neurotrophin-based therapeutics. Am J Psychiatry. 2011;168:114–6.
Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7–23.
Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–64.
Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–8.
Cao C, Rioult-Pedotti MS, Migani P, Yu CJ, Tiwari R, Parang K, et al. Impairment of TrkB-PSD-95 signaling in Angelman syndrome. PLoS Biol. 2013;11:e1001478.
Lau KA, Yang X, Rioult-Pedotti MS, Tang S, Appleman M, Zhang J, et al. A PSD-95 peptidomimetic mitigates neurological deficits in a mouse model of Angelman syndrome. Prog Neurobiol. 2023;230:102513.
Yang X, Huang YA, Marshall J. Targeting TrkB-PSD-95 coupling to mitigate neurological disorders. Neural Regen Res. 2025;20:715–24.
Piserchio A, Salinas GD, Li T, Marshall J, Spaller MR, Mierke DF. Targeting specific PDZ domains of PSD-95; structural basis for enhanced affinity and enzymatic stability of a cyclic peptide. Chem Biol. 2004;11:469–73.
Naik MT, Naik N, Hu T, Wang SH, Marshall J. Structure-based design of peptidomimetic inhibitors of PSD-95 with improved affinity for the PDZ3 domain. FEBS Lett. 2024;598:233–41.
Zeng M, Shang Y, Araki Y, Guo T, Huganir RL, Zhang M. Phase Transition in Postsynaptic Densities Underlies Formation of Synaptic Complexes and Synaptic Plasticity. Cell. 2016;166:1163–75.e12.
Shi X, Zhou XZ, Chen G, Luo WF, Zhou C, He TJ, et al. Targeting the postsynaptic scaffolding protein PSD-95 enhances BDNF signaling to mitigate depression-like behaviors in mice. Sci Signal. 2024;17:eadn4556.
Li KR, Huan MJ, Yao J, Li JJ, Cao Y, Wang S, et al. Syn3, a newly developed cyclic peptide and BDNF signaling enhancer, ameliorates retinal ganglion cell degeneration in diabetic retinopathy. Protein Cell. 2024;14:pwae028.
Salameh TS, Rhea EM, Talbot K, Banks WA. Brain uptake pharmacokinetics of incretin receptor agonists showing promise as Alzheimer’s and Parkinson’s disease therapeutics. Biochem Pharmacol. 2020;180:114187.
Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 2013;78:785–98.
Yang N, Ng YH, Pang ZP, Sudhof TC, Wernig M. Induced neuronal cells: how to make and define a neuron. Cell Stem Cell. 2011;9:517–25.
Fenton TA, Petkova SP, Adhikari A, Silverman JL. Acute administration of lovastatin improved several metrics of gait in a mouse model of Angelman Syndrome. J Neurodev Disord. 2023:in press.
Silverman JL, Babineau BA, Oliver CF, Karras MN, Crawley JN. Influence of stimulant-induced hyperactivity on social approach in the BTBR mouse model of autism. Neuropharmacology. 2013;68:210–22.
Kazdoba TM, Leach PT, Yang M, Silverman JL, Solomon M, Crawley JN. Translational Mouse Models of Autism: Advancing Toward Pharmacological Therapeutics. Curr Top Behav Neurosci. 2016;28:1–52.
Silverman JL, Smith DG, Rizzo SJ, Karras MN, Turner SM, Tolu SS, et al. Negative allosteric modulation of the mGluR5 receptor reduces repetitive behaviors and rescues social deficits in mouse models of autism. Sci Transl Med. 2012;4:131ra51.
Copping NA, Christian SGB, Ritter DJ, Islam MS, Buscher N, Zolkowska D, et al. Neuronal overexpression of Ube3a isoform 2 causes behavioral impairments and neuroanatomical pathology relevant to 15q11.2-q13.3 duplication syndrome. Hum Mol Genet. 2017;26:3995–4010.
Silverman JL, Crawley JN. The promising trajectory of autism therapeutics discovery. Drug Discov Today. 2014;19:838–44.
Silverman JL, Nithianantharajah J, Der-Avakian A, Young JW, Sukoff Rizzo SJ. Lost in translation: At the crossroads of face validity and translational utility of behavioral assays in animal models for the development of therapeutics. Neurosci Biobehav Rev. 2020;116:452–53.
Sukoff Rizzo SJ. The essential role of animal models in the advancement of our understanding of human behaviors: A Commentary on the Special issue on the 30th Anniversary of the International Behavioral Neuroscience Society (IBNS). Neurosci Biobehav Rev. 2023;149:105182.
Sukoff Rizzo SJ, Anderson LC, Green TL, McGarr T, Wells G, Winter SS. Assessing Healthspan and Lifespan Measures in Aging Mice: Optimization of Testing Protocols, Replicability, and Rater Reliability. Curr Protoc Mouse Biol. 2018;8:e45.
Sukoff Rizzo SJ, Homanics G, Schaeffer DJ, Schaeffer L, Park JE, Oluoch J, et al. Bridging the rodent to human translational gap: Marmosets as model systems for the study of Alzheimer’s disease. Alzheimers Dement. 2023;9:e12417.
Sukoff Rizzo SJ, Masters A, Onos KD, Quinney S, Sasner M, Oblak A, et al. Improving preclinical to clinical translation in Alzheimer’s disease research. Alzheimers Dement. 2020;6:e12038.
Sukoff Rizzo SJ, McTighe S, McKinzie DL. Genetic Background and Sex: Impact on Generalizability of Research Findings in Pharmacology Studies. Handb Exp Pharmacol. 2020;257:147–62.
Sukoff Rizzo SJ, Silverman JL. Methodological Considerations for Optimizing and Validating Behavioral Assays. Curr Protoc Mouse Biol. 2016;6:364–79.
Gompers AL, Su-Feher L, Ellegood J, Copping NA, Riyadh MA, Stradleigh TW, et al. Germline Chd8 haploinsufficiency alters brain development in mouse. Nat Neurosci. 2017;20:1062–73.
Haigh JL, Adhikari A, Copping NA, Stradleigh T, Wade AA, Catta-Preta R, et al. Deletion of a non-canonical regulatory sequence causes loss of Scn1a expression and epileptic phenotypes in mice. Genome Med. 2021;13:69.
Fenton TA, Haouchine OY, Hallam EB, Smith EM, Jackson KC, Rahbarian D, et al. Hyperexcitability and translational phenotypes in a preclinical mouse model of SYNGAP1-related intellectual disability. Transl. Psychiatry. 2024;14:405.
Yang M, Bozdagi O, Scattoni ML, Wohr M, Roullet FI, Katz AM, et al. Reduced excitatory neurotransmission and mild autism-relevant phenotypes in adolescent Shank3 null mutant mice. J Neurosci. 2012;32:6525–41.
Adhikari A, Copping NA, Onaga B, Pride MC, Coulson RL, Yang M, et al. Cognitive deficits in the Snord116 deletion mouse model for Prader-Willi syndrome. Neurobiol Learn Mem. 2019;165:106874.
Dhamne SC, Silverman JL, Super CE, Lammers SHT, Hameed MQ, Modi ME, et al. Replicable in vivo physiological and behavioral phenotypes of the Shank3B null mutant mouse model of autism. Mol Autism. 2017;8:26.
Silverman JL, Pride MC, Hayes JE, Puhger KR, Butler-Struben HM, Baker S, et al. GABAB Receptor Agonist R-Baclofen Reverses Social Deficits and Reduces Repetitive Behavior in Two Mouse Models of Autism. Neuropsychopharmacology. 2015;40:2228–39.
Weeber EJ, Jiang YH, Elgersma Y, Varga AW, Carrasquillo Y, Brown SE, et al. Derangements of hippocampal calcium/calmodulin-dependent protein kinase II in a mouse model for Angelman mental retardation syndrome. J Neurosci. 2003;23:2634–44.
DeVos SL, Goncharoff DK, Chen G, Kebodeaux CS, Yamada K, Stewart FR, et al. Antisense reduction of tau in adult mice protects against seizures. J Neurosci. 2013;33:12887–97.
Van Erum J, Valkenburg F, Van Dam D, De Deyn PP. Pentylenetetrazole-induced Seizure Susceptibility in the Tau58/4 Transgenic Mouse Model of Tauopathy. Neuroscience. 2020;425:112–22.
Van Erum J, Van Dam D, De Deyn PP. PTZ-induced seizures in mice require a revised Racine scale. Epilepsy Behav. 2019;95:51–5.
Copping NA, Adhikari A, Petkova SP, Silverman JL. Genetic backgrounds have unique seizure response profiles and behavioral outcomes following convulsant administration. Epilepsy Behav. 2019;101:106547.
Adhikari A, Buchanan FKB, Fenton TA, Cameron DL, Halmai J, Copping NA, et al. Touchscreen cognitive deficits, hyperexcitability and hyperactivity in males and females using two models of Cdkl5 deficiency. Hum Mol Genet. 2022;31:3032–50.
Born HA, Martinez LA, Levine AT, Harris SE, Mehra S, Lee WL, et al. Early Developmental EEG and Seizure Phenotypes in a Full Gene Deletion of Ubiquitin Protein Ligase E3A Rat Model of Angelman Syndrome. eNeuro. 2021;8:1–16.
Chung L, Bey AL, Towers AJ, Cao X, Kim IH, Jiang YH. Lovastatin suppresses hyperexcitability and seizure in Angelman syndrome model. Neurobiol Dis. 2018;110:12–9.
Ciarlone SL, Grieco JC, D’Agostino DP, Weeber EJ. Ketone ester supplementation attenuates seizure activity, and improves behavior and hippocampal synaptic plasticity in an Angelman syndrome mouse model. Neurobiol Dis. 2016;96:38–46.
Ciarlone SL, Wang X, Rogawski MA, Weeber EJ. Effects of the synthetic neurosteroid ganaxolone on seizure activity and behavioral deficits in an Angelman syndrome mouse model. Neuropharmacology. 2017;116:142–50.
Gu B, Carstens KE, Judson MC, Dalton KA, Rougie M, Clark EP, et al. Ube3a reinstatement mitigates epileptogenesis in Angelman syndrome model mice. J Clin Invest. 2019;129:163–68.
Gu B, Zhu M, Glass MR, Rougie M, Nikolova VD, Moy SS, et al. Cannabidiol attenuates seizures and EEG abnormalities in Angelman syndrome model mice. J Clin Invest. 2019;129:5462–67.
Kumar V, Joshi T, Vatsa N, Singh BK, Jana NR. Simvastatin Restores HDAC1/2 Activity and Improves Behavioral Deficits in Angelman Syndrome Model Mouse. Front Mol Neurosci. 2019;12:289.
Schultz MN, Crawley JN. Evaluation of a TrkB agonist on spatial and motor learning in the Ube3a mouse model of Angelman syndrome. Learn Mem. 2020;27:346–54.
Bruinsma CF, Schonewille M, Gao Z, Aronica EM, Judson MC, Philpot BD, et al. Dissociation of locomotor and cerebellar deficits in a murine Angelman syndrome model. J Clin Invest. 2015;125:4305–15.
van Woerden GM, Harris KD, Hojjati MR, Gustin RM, Qiu S, de Avila Freire R, et al. Rescue of neurological deficits in a mouse model for Angelman syndrome by reduction of [alpha]CaMKII inhibitory phosphorylation. Nat Neurosci. 2007;10:280–82.
Aleksandrova LR, Phillips AG. Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics. Trends Pharm Sci. 2021;42:929–42.
Casarotto PC, Girych M, Fred SM, Kovaleva V, Moliner R, Enkavi G, et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell. 2021;184:1299–313.e19.
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–5.
Egawa K, Nakakubo S, Kimura S, Goto T, Manabe A, Shiraishi H. Flurothyl-induced seizure paradigm revealed higher seizure susceptibility in middle-aged Angelman syndrome mouse model. Brain Dev. 2021;43:515–20.
Cruz E, Descalzi G, Steinmetz A, Scharfman HE, Katzman A, Alberini CM. CIM6P/IGF-2 Receptor Ligands Reverse Deficits in Angelman Syndrome Model Mice. Autism Res. 2021;14:29–45.
Leclercq K, Kaminski RM. Genetic background of mice strongly influences treatment resistance in the 6 Hz seizure model. Epilepsia. 2015;56:310–8.
Matson LM, McCarren HS, Cadieux CL, Cerasoli DM, McDonough JH. The role of genetic background in susceptibility to chemical warfare nerve agents across rodent and non-human primate models. Toxicology. 2018;393:51–61.
Rossetti F, Rodrigues MC, Marroni SS, Fernandes A, Foresti ML, Romcy-Pereira RN, et al. Behavioral and EEG effects of GABAergic manipulation of the nigro-tectal pathway in the Wistar audiogenic rat (WAR) strain II: an EEG wavelet analysis and retrograde neuronal tracer approach. Epilepsy Behav. 2012;24:391–8.
Schulz JB, Hausmann L. Synaptopathies: synaptic dysfunction in neurological disorders - A review written by students for students, and a story of success for ISN schools. J Neurochem. 2016;138:783–4.
Lepeta K, Lourenco MV, Schweitzer BC, Martino Adami PV, Banerjee P, Catuara-Solarz S, et al. Synaptopathies: synaptic dysfunction in neurological disorders - A review from students to students. J Neurochem. 2016;138:785–805.
Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–6.
Acknowledgements
We thank the Angelman Syndrome community and the UC Davis MIND Institute for supporting this research. We also thank Dr. Nathaniel Hodgson (IDDRC Animal Behavior and Physiology Core) for performing microdialysis and Rory (Dallon) Martin for exceptional husbandry and attention to the mouse colonies of the Silverman Laboratory and the IDDRC mouse behavioral core at the MIND Institute at UC Davis School of Medicine.
Funding
This work was supported by the National Institutes of Health [R01NS097808 (JLS), R01NS094440 (JM), R21MH104252 (JM), the Harrington Discovery Institute (JM), the MIND Institute’s Intellectual and Developmental Disabilities Resource Center (IDDRC), Grant/Award P50 HD103526 (PI, Abbeduto) and by generous funding from the Foundation for Angelman Syndrome Therapeutics (JM and XY)]. We also thank the IDDRC Animal Behavior and Physiology Core, funded by NIH/NICHD P50 HD105351.
Author information
Authors and Affiliations
Contributions
JLS and JM designed the study. EZH and ERM generated the mice through paternal and maternal specific breeding lines and performed genotyping to identify mice for groups. EZH, KT, and AYY performed the behavioral experiments and subsequent analyses. XY and YAH conducted the biochemistry and signaling studies in the iPSC cells. MSP conducted the LTP analysis. MN completed the structural biology analysis. KH, MAE and WAB conducted the pharmacokinetic studies. JM and JLS supervised the study and interpretations of data. EZH, JM, and JLS drafted the initial manuscript. All authors included valuable comments and edits to the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huie, E.Z., Yang, X., Rioult-Pedotti, M.S. et al. Peptidomimetic inhibitors targeting TrkB/PSD-95 signaling improves cognition and seizure outcomes in an Angelman Syndrome mouse model. Neuropsychopharmacol. 50, 772–782 (2025). https://doi.org/10.1038/s41386-024-02020-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-024-02020-z