Abstract
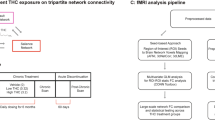
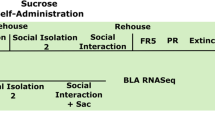
Although chronic cannabis use during adolescence can alter brain function and impair complex behavioral processes, it is unclear whether such deficits persist into adulthood. Using a coordinated awake neuroimaging and behavioral approach in nonhuman primates, we addressed this issue by examining the impact of chronic adolescent exposure to Δ9-tetrahydrocannabinol (THC) on brain functional connectivity and motivational processes during early adulthood. Female and male squirrel monkeys (n = 23) were treated daily for 6 months during adolescence with vehicle or either a low (0.32 mg/kg) or high dose (3.2 mg/kg) of THC. Regional homogeneity and seed-to-whole-brain functional connectivity were analyzed prior to, during, and following discontinuation of chronic treatment to examine changes in regions implicated in reward processing. Subsequently, motivation and reward sensitivity in these subjects, now young adults, were evaluated in economic demand studies by determining the relationship between escalating response requirements and consumption of differing magnitudes of a palatable food reinforcer. Results show that adolescent THC exposure led to persistent alterations in mOFC, caudate, and ventral striatum whole-brain connectivity. Moreover, subjects treated with vehicle during adolescence displayed an orderly and expected inverse relationship between reward magnitude and demand elasticity, whereas THC-treated subjects exhibited dosage-dependent disorder in reward sensitivity and motivational deficits. Changes in neural circuitry (local connectivity in ventral striatum and whole brain connectivity in mOFC) and economic demand were correlated with indices of reward sensitivity in vehicle- but not THC-treated subjects. Taken together, these data indicate that chronic adolescent THC exposure produced long-lasting neurocognitive abnormalities in reward processing.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bara A, Ferland JN, Rompala G, Szutorisz H, Hurd YL. Cannabis and synaptic reprogramming of the developing brain. Nat Rev Neurosci. 2021;22:423–38.
Brumback T, Castro N, Jacobus J, Tapert S. Effects of marijuana use on brain structure and function: neuroimaging findings from a neurodevelopmental perspective. Int Rev Neurobiol. 2016;129:33–65.
Ferland JN, Ellis RJ, Betts G, Silveira MM, de Firmino JB, Winstanley CA, et al. Long-term outcomes of adolescent THC exposure on translational cognitive measures in adulthood in an animal model and computational assessment of human data. JAMA Psychiatry. 2023;80:66–76.
Lichenstein SD, Manco N, Cope LM, Egbo L, Garrison KA, Hardee J, et al. Systematic review of structural and functional neuroimaging studies of cannabis use in adolescence and emerging adulthood: evidence from 90 studies and 9441 participants. Neuropsychopharmacology. 2022;47:1000–28.
Rubino T, Parolaro D. The impact of exposure to cannabinoids in adolescence: insights from animal models. Biol Psychiatry. 2016;79:578–85.
Wilson W, Mathew R, Turkington T, Hawk T, Coleman RE, Provenzale J. Brain morphological changes and early marijuana use: a magnetic resonance and positron emission tomography study. J Addict Dis. 2000;19:1–22.
Camchong J, Lim KO, Kumra S. Adverse effects of cannabis on adolescent brain development: a longitudinal study. Cereb Cortex. 2017;27:1922–30.
Cheng H, Skosnik PD, Pruce BJ, Brumbaugh MS, Vollmer JM, Fridberg DJ, et al. Resting state functional magnetic resonance imaging reveals distinct brain activity in heavy cannabis users - a multi-voxel pattern analysis. J Psychopharmacol. 2014;28:1030–40.
Houck JM, Bryan AD, Feldstein Ewing SW. Functional connectivity and cannabis use in high-risk adolescents. Am J Drug Alcohol Abus. 2013;39:414–23.
Lorenzetti V, Hoch E, Hall W. Adolescent cannabis use, cognition, brain health and educational outcomes: A review of the evidence. Eur Neuropsychopharmacol. 2020;36:169–80.
Batalla A, Bhattacharyya S, Yücel M, Fusar-Poli P, Crippa JA, Nogué S, et al. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS One. 2013;8:e55821.
Gleason KA, Birnbaum SG, Shukla A, Ghose S. Susceptibility of the adolescent brain to cannabinoids: long-term hippocampal effects and relevance to schizophrenia. Transl Psychiatry. 2012;2:e199.
Jacobus J, Goldenberg D, Wierenga CE, Tolentino NJ, Liu TT, Tapert SF. Altered cerebral blood flow and neurocognitive correlates in adolescent cannabis users. Psychopharmacology. 2012;222:675–84.
Rubino T, Realini N, Braida D, Guidi S, Capurro V, Viganò D, et al. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19:763–72.
Gruber SA, Dahlgren MK, Sagar KA, Gönenc A, Lukas SE. Worth the wait: effects of age of onset of marijuana use on white matter and impulsivity. Psychopharmacol. 2013;231:1455–65.
Rubia K. Functional brain imaging across development. Eur Child Adolesc Psychiatry. 2013;22:719–31.
Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2:859–61.
Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–31.
Levine A, Clemenza K, Rynn M, Lieberman J. Evidence for the risks and consequences of adolescent cannabis exposure. J Am Acad Child Adolesc Psychiatry. 2017;56:214–25.
Lisdahl KM, Wright NE, Kirchner-Medina C, Maple KE, Shollenbarger S. Considering cannabis: the effects of regular cannabis use on neurocognition in adolescents and young adults. Curr Addict Rep. 2014;1:144–56.
Raphael D. Adolescence as a gateway to adult health outcomes. Maturitas. 2013;75:137–41.
McGlothlin WH, West LJ. The marihuana problem: an overview. Am J Psychiatry. 1968;125:126–34.
Pacheco-Colón I, Limia JM, Gonzalez R. Nonacute effects of cannabis use on motivation and reward sensitivity in humans: A systematic review. Psychol Addict Behav. 2018;32:497–507.
Lac A, Luk JW. Testing the Amotivational Syndrome: Marijuana use longitudinally predicts lower self-efficacy even after controlling for demographics, personality, and alcohol and cigarette use. Prev Sci. 2018;19:117–26.
Martz ME, Trucco EM, Cope LM, Hardee JE, Jester JM, Zucker RA, et al. Association of marijuana use with blunted nucleus accumbens response to reward anticipation. JAMA Psychiatry. 2016;73:838–44.
Paule MG, Allen RR, Bailey JR, Scallet AC, Ali SF, Brown RM, et al. Chronic marijuana smoke exposure in the rhesus monkey. II: Effects on progressive ratio and conditioned position responding. J Pharm Exp Ther. 1992;260:210–22.
Halbout B, Hutson C, Hua L, Inshishian V, Mahler SV, Ostlund SB. Long-term effects of THC exposure on reward learning and motivated behavior in adolescent and adult male rats. Psychopharmacol. 2023;240:1151–67.
Kangas BD, Leonard MZ, Shukla VG, Alapafuja SO, Nikas SP, Makriyannis A, et al. Comparisons of D9-Tetrahydrocannabinol and anandamide on a battery of cognition-related behavior in nonhuman primates. J Pharm Exp Ther. 2016;357:125–33.
Kruse LC, Cao JK, Viray K, Stella N, Clark JJ. Voluntary oral consumption of Δ9-tetrahydrocannabinol by adolescent rats impairs reward-predictive cue behaviors in adulthood. Neuropsychopharmacology. 2019;44:1406–14.
Orihuel J, Capellán R, Roura-Martínez D, Ucha M, Ambrosio E, Higuera-Matas A. Δ 9-tetrahydrocannabinol during adolescence reprograms the nucleus accumbens transcriptome, affecting reward processing, impulsivity, and specific aspects of cocaine addiction-like behavior in a sex-dependent manner. Int J Neuropsychopharmacol. 2021;24:920–33.
Pushkin AN, Eugene AJ, Lallai V, Torres-Mendoza A, Fowler JP, Chen E, et al. Cannabinoid and nicotine exposure during adolescence induces sex-specific effects on anxiety- and reward-related behaviors during adulthood. PLoS One. 201931;14:e0211346.
Schoch H, Huerta MY, Ruiz CM, Farrell MR, Jung KM, Huang JJ, et al. Adolescent cannabinoid exposure effects on natural reward seeking and learning in rats. Psychopharmacology. 2018;235:121–34.
Branch MN, Dearing ME, Lee DM. Acute and chronic effects of delta 9- tetrahydrocannabinol on complex behavior of squirrel monkeys. Psychopharmacology. 1980;71:247–56.
Justinova Z, Mascia P, Wu HQ, Secci ME, Redhi GH, Panlilio LV, et al. Reducing cannabinoid abuse and preventing relapse by enhancing endogenous brain levels of kynurenic acid. Nat Neurosci. 2013;16:1652–61.
Justinova Z, Tanda G, Redhi GH, Goldberg SR. Self-administration of delta9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology. 2003;169:135–40.
Kangas BD, Delatte MS, Vemuri VK, Thakur GA, Nikas SP, Subramanian KV, et al. Cannabinoid discrimination and antagonism by CB(1) neutral and inverse agonist antagonists. J Pharm Exp Ther. 2013;344:561–7.
Leonard MZ, Alapafuja SO, Ji L, Shukla VG, Liu Y, Nikas SP, et al. Cannabinoid CB1 discrimination: effects of endocannabinoids and catabolic enzyme inhibitors. J Pharm Exp Ther. 2017;363:314–23.
Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, Piomelli D, et al. The endogenous cannabinoid anandamide produces delta-9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. J Pharm Exp Ther. 2007;321:370–80.
Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3:1073–4.
Withey SL, Kangas BD, Charles S, Gumbert AB, Eisold JE, George SR, et al. Effects of daily Δ9-Tetrahydrocannabinol (THC) alone or combined with cannabidiol (CBD) on cognition-based behavior and activity in adolescent nonhuman primates. Drug Alcohol Depend. 2021;221:108629.
Brady AG. Research techniques for the squirrel monkey (Saimiri sp.). ILAR J. 2000;41:10–8.
National Research Council (US) Committee for the update of the guide for the care and use of laboratory animals. Guide for the Care and Use of Laboratory Animals. (8th ed.). 2011;National Academies Press (US).
Kangas BD, Bergman J. A novel touch-sensitive apparatus for behavioral studies in unrestrained squirrel monkeys. J Neurosci Meth. 2012;209:331–6.
Kangas BD, Bergman J. Touchscreen technology in the study of cognition-related behavior. Behav Pharm. 2017;28:623–9.
Withey SL, Bergman J, Huestis MA, George SR, Madras BK. THC and CBD blood and brain concentrations following daily administration to adolescent primates. Drug Alcohol Depend. 2020;213:108129.
Yassin W, de Moura FB, Withey SL, Cao L, Kangas BD, Bergman J, et al. Resting-state networks of awake adolescent and adult squirrel monkeys using ultra-high field (9.4T) functional magnetic resonance imaging. eNeuro. 2024;11:ENEURO.0173-23.2024.
Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115:186–98.
Esteban O, Birman D, Schaer M, Koyejo OO, Poldrack RA, Gorgolewski KJ. MRIQC: Advancing the automatic prediction of image quality in MRI from unseen sites. PLoS One. 2017;12:e0184661.
Schilling K, Gao Y, Stepniewska I, Choe AS, Landman BA, Anderson AW. Reproducibility and variation of diffusion measures in the squirrel monkey brain, in vivo and ex vivo. Magn Reson Imaging. 2017;35:29–38.
Zuo XN, Xu T, Jiang L, Yang Z, Cao XY, He Y, et al. Toward reliable characterization of functional homogeneity in the human brain: preprocessing, scan duration, imaging resolution and computational space. Neuroimage. 2013;65:374–86.
Yuan R, Nechvatal JM, Buckmaster CL, Ayash S, Parker KJ, Schatzberg AF, et al. Long-term effects of intermittent early life stress on primate prefrontal-subcortical functional connectivity. Neuropsychopharmacology. 2021;46:1348–56.
Lee AG, Nechvatal JM, Shen B, Buckmaster CL, Levy MJ, Chin FT, et al. Striatal dopamine D2/3 receptor regulation by stress inoculation in squirrel monkeys. Neurobiol Stress. 2016;3:68–73.
Zuo XN, Xing XX. Test-retest reliabilities of resting-state FMRI measurements in human brain functional connectomics: a systems neuroscience perspective. Neurosci Biobehav Rev. 2014;45:100–18.
Kohut SJ, Cao L, Mintzopolous D, Jiang S, Nikas SP, Makriyannis A, et al. Effects of cannabinoid exposure on short-term memory and medial orbitofrontal cortex function and chemistry in adolescent female rhesus macaques. Front Neurosci. 2022;16:998351.
Strickland JC, Lacy RT. Behavioral economic demand as a unifying language for addiction science: promoting collaboration and integration of animal and human models. Exp Clin Psychopharmacol. 2020;28:404–16.
Peters J, Büchel C. Neural representations of subjective reward value. Behav Brain Res. 2010;213:135–41.
Bloomfield MAP, Hindocha C, Green SF, Wall MB, Lees R, Petrilli K, et al. The neuropsychopharmacology of cannabis: A review of human imaging studies. Pharmacol Ther 2019;195:132–61.
Sehl H, Terrett G, Greenwood LM, Kowalczyk M, Thomson H, Poudel G, et al. Patterns of brain function associated with cannabis cue-reactivity in regular cannabis users: a systematic review of fMRI studies. Psychopharmacology. 2021;238:2709–28.
Zimmermann K, Yao S, Heinz M, Zhou F, Dau W, Banger M, et al. Altered orbitofrontal activity and dorsal striatal connectivity during emotion processing in dependent marijuana users after 28 days of abstinence. Psychopharmacology. 2018;235:849–59.
Jenni NL, Rutledge G, Floresco SB. Distinct medial orbitofrontal-striatal circuits support dissociable component processes of risk/reward decision-making. J Neurosci. 2022;42:2743–55.
Gourley SL, Zimmermann KS, Allen AG, Taylor JR. The medial orbitofrontal cortex regulates sensitivity to outcome value. J Neurosci. 2016;36:4600–13.
Burton AC, Kashtelyan V, Bryden DW, Roesch MR. Increased firing to cues that predict low-value reward in the medial orbitofrontal cortex. Cereb Cortex. 2014;24:3310–21.
Chou S, Ranganath T, Fish KN, Lewis DA, Sweet RA. Cell type specific cannabinoid CB1 receptor distribution across the human and non-human primate cortex. Sci Rep. 2022;12:9605.
Schwitzer T, Schwan R, Angioi-Duprez K, Ingster-Moati I, Lalanne L, Giersch A, et al. The cannabinoid system and visual processing: a review on experimental findings and clinical presumptions. Eur Neuropsychopharmacol. 2015;25:100–12.
Mato S, Del Olmo E, Pazos A. Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. Eur J Neurosci. 2003;17:1747–54.
Klumpers LE, Cole DM, Khalili-Mahani N, Soeter RP, Te Beek ET, Rombouts SA, et al. Manipulating brain connectivity with δ⁹-tetrahydrocannabinol: a pharmacological resting state FMRI study. Neuroimage. 2012;63:1701–11.
Winton-Brown TT, Allen P, Borgwardt BhattacharyyaS, Fusar-Poli SJ, Crippa P, McGuire JA. PK. Modulation of auditory and visual processing by delta-9-tetrahydrocannabinol (THC): A multimodal neuroimaging study. Neuropsychopharmacology. 2011;36:1340–8.
Chang L, Yakupov R, Cloak C, Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006;129:1096–112.
Blest-Hopley G, Giampietro V, Bhattacharyya S. Regular cannabis use is associated with altered activation of central executive and default mode networks even after prolonged abstinence in adolescent users: results from a complementary meta-analysis. Neurosci Biobehav Rev. 2019;96:45–55.
Diekhof EK, Nerenberg L, Falkai P, Dechent P, Baudewig J, Gruber O. Impulsive personality and the ability to resist immediate reward: an fMRI study examining interindividual differences in the neural mechanisms underlying self-control. Hum Brain Mapp. 2012;33:2768–84.
Lopez-Gamundi P, Yao YW, Chong TT, Heekeren HR, Mas-Herrero E, Marco-Pallarés J. The neural basis of effort valuation: a meta-analysis of functional magnetic resonance imaging studies. Neurosci Biobehav Rev. 2021;131:1275–87.
Hur KH, Meisler SL, Yassin W, Frederick BB, Kohut SJ. Prefrontal-limbic circuitry is associated with reward sensitivity in nonhuman primates. Biol Psychiatry. 2024;96:473–85.
Silverman MH, Jedd K, Luciana M. Neural networks involved in adolescent reward processing: an activation likelihood estimation meta-analysis of functional neuroimaging studies. Neuroimage. 2015;122:427–39.
Galván A, McGlennen KM. Enhanced striatal sensitivity to aversive reinforcement in adolescents versus adults. J Cogn Neurosci. 2013;25:284–96.
Deshpande HU, Kohut SJ. Age-related development in prefrontal-subcortical resting-state functional connectivity in nonhuman primates. 2023;bioRxiv. 2023;07:549741.
Albaugh MD, Ottino-Gonzalez J, Sidwell A, Lepage C, Juliano A, Owens MM, et al. Association of cannabis use during adolescence with neurodevelopment. JAMA Psychiatry. 2021;78:1–11.
Funding
This project was supported by R01-DA047575 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
BDK, SLW, RDS, JB, and SJK designed the research, BDK, SLW, and SJK performed the research, BDK, HUD, and SJK analyzed the data, and BDK, HUD, SLW, RDS, JB, SJK wrote the paper.
Corresponding author
Ethics declarations
Competing interests
Over the past 3 years, BDK has had sponsored research agreements with BlackThorn Therapeutics, Compass Pathways, Delix Therapeutics, Engrail Therapeutics, Neurocrine Biosciences, and Takeda Pharmaceuticals. No funding from these entities was used to support the current work. All other authors have no conflicts of interest or relevant disclosures.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kangas, B.D., Deshpande, H.U., Withey, S.L. et al. Chronic Δ9-tetrahydrocannabinol exposure in adolescent nonhuman primates: persistent abnormalities in economic demand and brain functional connectivity. Neuropsychopharmacol. 50, 576–585 (2025). https://doi.org/10.1038/s41386-024-02024-9
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-024-02024-9