Abstract
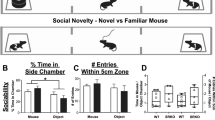
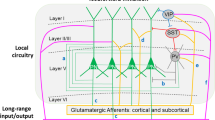
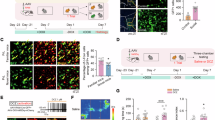
Emotion recognition is fundamental for effective social interactions among conspecifics. Impairments in affective state processing underlie several neuropsychiatric disorders, including schizophrenia, although the neurobiological substrate of these deficits remains unknown. We investigated the impact of early NMDA receptor hypofunction on socio-affective behaviors. Male mice lacking NMDA receptors in GABAergic interneurons of cerebral and hippocampal cortices from an early postnatal age (interNMDAr-KO mutants) were evaluated in affective state discrimination, social preference and social novelty preference, hierarchy and dominance, aggression and territoriality, and long-term social interaction. We show that interNMDAr-KO mice failed to discriminate conspecifics based on their affective states, unlike control littermates, while exhibiting an intact preference for social stimuli over inanimate objects. This discrimination deficit was observed regardless of whether affective valences were manipulated positively or negatively, via a palatable reward or social defeat, respectively. Additionally, interNMDAr-KO mice failed to establish a normal social hierarchy, consistently assuming subordinate roles against control littermates, and presented an abnormal response to conspecifics in the resident-intruder test. Finally, mice lacking NMDA receptors in GABAergic interneurons exhibited social withdrawal following exposure to unfamiliar conspecifics in a custom setting designed to monitor social behavior over extended time periods. This deficit was reversed by subchronic clozapine treatment. Our study thoroughly assessed the impact of a pathophysiological manipulation relevant to schizophrenia on social behavior in mice. Overall, this study provides evidence demonstrating that altered NMDAr-dependent development of cortical and hippocampal interneurons impairs affective state discrimination and leads to deficits in social functioning and long-term sociality.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bartal IBA, Decety J, Mason P. Empathy and pro-social behavior in rats. Science. 2011;334:1427–30.
Zhang M, Wu YE, Jiang M, Hong W. Cortical regulation of helping behaviour towards others in pain. Nature. 2024;626:136–44.
Burkett JP, Andari E, Johnson ZV, Curry DC, De Waal FBM, Young LJ. Oxytocin-dependent consolation behavior in rodents. Science. 2016;351:375–8.
Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, et al. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci. 2010;13:482–8.
Rice GE, Gainer P. ‘Altruism’ in the albino rat. J Comp Physiol Psychol. 1962;55:123–5.
Church RM. Emotional reactions of rats to the pain of others. J Comp Physiol Psychol. 1959;52:132–4.
Riess D. Vicarious conditioned acceleration: successful observational learning of an aversive Pavlovian stimulus contingency. J Exp Anal Behav. 1972;18:181–6.
Rogers-Carter MM, Varela JA, Gribbons KB, Pierce AF, McGoey MT, Ritchey M, et al. Insular cortex mediates approach and avoidance responses to social affective stimuli. Nat Neurosci. 2018;21:404–14.
Ferretti V, Maltese F, Contarini G, Nigro M, Bonavia A, Huang H, et al. Oxytocin signaling in the central amygdala modulates emotion discrimination in mice. Curr Biol. 2019;29:1938–1953.e6.
Scheggia D, Managò F, Maltese F, Bruni S, Nigro M, Dautan D, et al. Somatostatin interneurons in the prefrontal cortex control affective state discrimination in mice. Nat Neurosci. 2020;23:47–60.
Savla GN, Vella L, Armstrong CC, Penn DL, Twamley EW. Deficits in domains of social cognition in schizophrenia: a meta-analysis of the empirical evidence. Schizophr Bull. 2013;39:979–92.
Green MF, Horan WP, Lee J. Social cognition in schizophrenia. Nat Rev Neurosci. 2015;16:620–31.
Mandal MK, Habel U, Gur RC. Facial expression-based indicators of schizophrenia: evidence from recent research. Schizophr Res. 2023;252:335–44.
Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 2006;32:S44–S63.
Halverson TF, Orleans-Pobee M, Merritt C, Sheeran P, Fett AK, Penn DL. Pathways to functional outcomes in schizophrenia spectrum disorders: meta-analysis of social cognitive and neurocognitive predictors. Neurosci Biobehav Rev. 2019;105:212–9.
Marder SR, Galderisi S. The current conceptualization of negative symptoms in schizophrenia. World Psychiatry. 2017;16:14–24.
Green MF, Horan WP, Lee J, McCleery A, Reddy LF, Wynn JK. Social disconnection in schizophrenia and the general community. Schizophr Bull. 2018;44:242–9.
Dodell-Feder D, Tully LM, Hooker CI. Social impairment in schizophrenia: new approaches for treating a persistent problem. Curr Opin Psychiatry. 2015;28:236–42.
Lemmers-Jansen I, Velthorst E, Fett AK. The social cognitive and neural mechanisms that underlie social functioning in individuals with schizophrenia—a review. Transl Psychiatry. 2023;13:327.
Harrison PJ, Bannerman DM. GRIN2A (NR2A): a gene contributing to glutamatergic involvement in schizophrenia. Mol Psychiatry. 2023;28:3568–72.
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214.
Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15.
Coyle JT, Brad Ruzicka W, Balu DT. Fifty years of research on schizophrenia: the ascendance of the glutamatergic synapse. Am J Psychiatry. 2020;177:1119–28.
Singh T, Poterba T, Curtis D, Akil H, Al Eissa M, Barchas JD, et al. Rare coding variants in ten genes confer substantial risk for schizophrenia. Nature. 2022;604:509–16.
Nakazawa K, Sapkota K. The origin of NMDA receptor hypofunction in schizophrenia. Pharmacol Ther. 2020;205:107426.
Weickert CS, Fung SJ, Catts VS, Schofield PR, Allen KM, Moore LT, et al. Molecular evidence of N-methyl-D-aspartate receptor hypofunction in schizophrenia. Mol Psychiatry. 2013;18:1185–92.
Gao WJ, Yang SS, Mack NR, Chamberlin LA. Aberrant maturation and connectivity of prefrontal cortex in schizophrenia-contribution of NMDA receptor development and hypofunction. Mol Psychiatry. 2022;27:731–43.
Uliana DL, Lisboa JRF, Gomes FV, Grace AA. The excitatory-inhibitory balance as a target for the development of novel drugs to treat schizophrenia. Biochem Pharmacol. 2024;228:116298.
Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, et al. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 2012;62:1574–83.
Powell SB, Sejnowski TJ, Behrens MM. Behavioral and neurochemical consequences of cortical oxidative stress on parvalbumin-interneuron maturation in rodent models of schizophrenia. Neuropharmacology. 2012;62:1322–31.
Marín O. Parvalbumin interneuron deficits in schizophrenia. Eur Neuropsychopharmacol. 2024;82:44–52.
Rotaru DC, Lewis DA, Gonzalez-Burgos G. The role of glutamatergic inputs onto parvalbumin-positive interneurons: relevance for schizophrenia. Rev Neurosci. 2012;23:97–109.
Sullivan EM, O’Donnell P. Inhibitory interneurons, oxidative stress, and schizophrenia. Schizophr Bull. 2012;38:373–6.
Cooper MA, Koleske AJ. ErbB4 localization to interneurons: clearer insights into schizophrenia pathology. Biol Psychiatry. 2011;70:602–3.
Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83.
Alvarez RJ, Pafundo DE, Zold CL, Belforte JE. Interneuron NMDA receptor ablation induces hippocampus-prefrontal cortex functional hypoconnectivity after adolescence in a mouse model of schizophrenia. J Neurosci. 2020;40:3304–17.
Nakao K, Jeevakumar V, Jiang SZSZ, Fujita Y, Diaz NBNBNB, Annan CAPCAP, et al. Schizophrenia-like dopamine release abnormalities in a mouse model of NMDA receptor hypofunction. Schizophr Bull. 2019;45:138–47.
Pafundo DE, Pretell Annan CA, Fulginiti NM, Belforte JE. Early NMDA receptor ablation in interneurons causes an activity-dependent E/I imbalance in vivo in prefrontal cortex pyramidal neurons of a mouse model useful for the study of schizophrenia. Schizophr Bull. 2021;47:1300–9.
Nakao K, Nakazawa K. Brain state-dependent abnormal LFP activity in the auditory cortex of a schizophrenia mouse model. Front Neurosci. 2014;8:168.
Jiang Z, Rompala GR, Zhang S, Cowell RM, Nakazawa K. Social isolation exacerbates schizophrenia-like phenotypes via oxidative stress in cortical interneurons. Biol Psychiatry. 2013;73:1024–34.
Qin L, Ma K, Wang ZJ, Hu Z, Matas E, Wei J, et al. Social deficits in Shank3-deficient mouse models of autism are rescued by histone deacetylase (HDAC) inhibition. Nat Neurosci. 2018;21:564–75.
Peñagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–46.
Moy SS, Ghashghaei HT, Nonneman RJ, Weimer JM, Yokota Y, Lee D, et al. Deficient NRG1-ERBB signaling alters social approach: relevance to genetic mouse models of schizophrenia. J Neurodev Disord. 2009;1:302.
Duncan GE, Moy SS, Perez A, Eddy DM, Zinzow WM, Lieberman JA, et al. Deficits in sensorimotor gating and tests of social behavior in a genetic model of reduced NMDA receptor function. Behav Brain Res. 2004;153:507–19.
Jabarin R, Netser S, Wagner S. Beyond the three-chamber test: toward a multimodal and objective assessment of social behavior in rodents. Mol Autism. 2022;13:41.
Fan Z, Zhu H, Zhou T, Wang S, Wu Y, Hu H. Using the tube test to measure social hierarchy in mice. Nat Protoc. 2019;14:819–31.
Koolhaas JM, Coppens CM, de Boer SF, Buwalda B, Meerlo P, Timmermans PJA. The resident-intruder paradigm: a standardized test for aggression, violence and social stress. J Vis Exp. 2013;77:4367.
Vasconcelos M, Stein DJ, de Almeida RMM. Social defeat protocol and relevant biomarkers, implications for stress response physiology, drug abuse, mood disorders and individual stress vulnerability: a systematic review of the last decade. Trends Psychiatry Psychother. 2015;37:51–66.
Berridge KC, Robinson TE. Liking, wanting and the incentive-sensitization theory of addiction. Am Psychol. 2016;71:670.
Krakenberg V, Siestrup S, Palme R, Kaiser S, Sachser N, Richter SH. Effects of different social experiences on emotional state in mice. Sci Rep. 2020;10:15255.
Powell SB, Swerdlow NR. The relevance of animal models of social isolation and social motivation for understanding schizophrenia: review and future directions. Schizophr Bull. 2023;49:1112–26.
Millan MJ, Agid Y, Brüne M, Bullmore ET, Carter CS, Clayton NS, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11:141–68.
Yang M, Silverman JL, Crawley JN. Automated three-chambered social approach task for mice. Curr Protoc Neurosci. 2011;56:8.26.1–8.26.16.
Porcelli S, Van Der Wee N, van der Werff S, Aghajani M, Glennon JC, van Heukelum S, et al. Social brain, social dysfunction and social withdrawal. Neurosci Biobehav Rev. 2019;97:10–33.
Bicks LK, Peng M, Taub A, Akbarian S, Morishita H. An adolescent sensitive period for social dominance hierarchy plasticity is regulated by cortical plasticity modulators in mice. Front Neural Circuits. 2021;15:676308.
Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–14.
Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes, Brain Behav. 2004;3:287–302.
Crawley JN. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment Retard Dev Disabil Res Rev. 2004;10:248–58.
delPino I, García-Frigola C, Dehorter N, Brotons-Mas JR, Alvarez-Salvado E, MartínezdeLagrán M, et al. Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron. 2013;79:1152–68.
Cao W, Li JH, Lin S, Xia QQ, Du YL, Yang Q, et al. NMDA receptor hypofunction underlies deficits in parvalbumin interneurons and social behavior in neuroligin 3 R451C knockin mice. Cell Rep. 2022;41:111771.
Ma Q, Jiang L, Chen H, An D, Ping Y, Wang Y, et al. Histamine H2 receptor deficit in glutamatergic neurons contributes to the pathogenesis of schizophrenia. Proc Natl Acad Sci USA. 2023;120:e2207003120.
Golani I, Tadmor H, Buonanno A, Kremer I, Shamir A. Disruption of the ErbB signaling in adolescence increases striatal dopamine levels and affects learning and hedonic-like behavior in the adult mouse. Eur Neuropsychopharmacol. 2014;24:1808–18.
Takao K, Kobayashi K, Hagihara H, Ohira K, Shoji H, Hattori S, et al. Deficiency of schnurri-2, an MHC enhancer binding protein, induces mild chronic inflammation in the brain and confers molecular, neuronal, and behavioral phenotypes related to schizophrenia. Neuropsychopharmacology. 2013;38:1409–25.
Langford DJ, Crager SE, Shehzad Z, Smith SB, Sotocinal SG, Levenstadt JS, et al. Social modulation of pain as evidence for empathy in mice. Science. 2006;312:1967–70.
Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–77.
Mack NR, Bouras NN, Gao WJ. Prefrontal regulation of social behavior and related deficits: insights from rodent studies. Biol Psychiatry. 2024;96:85–94.
Bicks LK, Koike H, Akbarian S, Morishita H. Prefrontal cortex and social cognition in mouse and man. Front Psychol. 2015;6:1805.
Thorup A, Albert N, Bertelsen M, Petersen L, Jeppesen P, Le Quack P, et al. Gender differences in first-episode psychosis at 5-year follow-up-two different courses of disease? Results from the OPUS study at 5-year follow-up. Eur Psychiatry. 2014;29:44–51.
Lappin JM, Heslin M, Lomas B, Jones PB, Doody GA, Reininghaus UA, et al. Early sustained recovery following first episode psychosis: evidence from the AESOP10 follow-up study. Schizophr Res. 2018;199:341–5.
Grossman LS, Harrow M, Rosen C, Faull R, Strauss GP. Sex differences in schizophrenia and other psychotic disorders: a 20-year longitudinal study of psychosis and recovery. Compr Psychiatry. 2008;49:523.
Perry W, Moore D, Braff D. Gender differences on thought disturbance measures among schizophrenic patients. Am J Psychiatry. 1995;152:1298–301.
Saunders JA, Tatard-Leitman VM, Suh J, Billingslea EN, Roberts TP, Siegel SJ. Knockout of NMDA receptors in parvalbumin interneurons recreates autism-like phenotypes. Autism Res. 2013;6:69–77.
Billingslea EN, Tatard-Leitman VM, Anguiano J, Jutzeler CR, Suh J, Saunders JA, et al. Parvalbumin cell ablation of NMDA-R1 causes increased resting network excitability with associated social and self-care deficits. Neuropsychopharmacol. 2014;39:1603–13.
Ferri SL, Pallathra AA, Kim H, Dow HC, Raje P, McMullen M, et al. Sociability development in mice with cell-specific deletion of the NMDA receptor NR1 subunit gene. Genes Brain Behav. 2020;19:e12624.
Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:0878–90.
Carta I, Chen CH, Schott AL, Dorizan S, Khodakhah K. Cerebellar modulation of the reward circuitry and social behavior. Science. 2019;363:eaav0581.
Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, et al. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 2012;488:647–51.
Goldberg EM, Jeong HY, Kruglikov I, Tremblay R, Lazarenko RM, Rudy B. Rapid developmental maturation of neocortical FS cell intrinsic excitability. Cereb Cortex. 2011;21:666–82.
Doischer D, Hosp JA, Yanagawa Y, Obata K, Jonas P, Vida I, et al. Postnatal differentiation of basket cells from slow to fast signaling devices. J Neurosci. 2008;28:12956–68.
Millan MJ, Bales KL. Towards improved animal models for evaluating social cognition and its disruption in schizophrenia: the CNTRICS initiative. Neurosci Biobehav Rev. 2013;37:2166–80.
Acknowledgements
We thank Camila Zold, Noelia Weisstaub, and Amaicha Depino for their critical reading of the manuscript; Analia Lopez Diaz, Agostina Presta, Veronica Risso, Lucia Garbini, Andres Varani, and Micaela Buscema for technical assistance, and the GNS group for feedback on previous drafts. Illustrations were created with BioRender. We also thank the Rospaw Laboratory for their kind donation of clozapine.
Funding
This work was supported in part by Agencia Nacional de Promoción Científica y Tecnológica, Proyectos de Investigación Científicos y Tecnológicos PICT 2019-3626 [EPB], 2018-0581 and 2021-00324 [JEB]; Universidad de Buenos Aires, UBACYT 20020220300072BA [EPB, JEB]; Brain and Behavior Research Foundation, NARSAD Young Investigator Grant Award 2015-#23983 [EPB]; Pew Charitable Trusts Repatriation Grant [EPB]; Fundación Bunge y Born - Fundación Williams, Mario Hirsch Award [EPB]. All authors were supported by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and the Universidad de Buenos Aires, Argentina.
Author information
Authors and Affiliations
Contributions
JMU, EPB & JEB designed the study. JMU & MGA performed the experiments and collected data. JMU, MGA, EPB & JEB analyzed the data. JMU, EPB & JEB wrote the manuscript. JMU, MGA, EPB & JEB reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Uehara, J.M., Gomez Acosta, M., Bello, E.P. et al. Early postnatal NMDA receptor ablation in cortical interneurons impairs affective state discrimination and social functioning. Neuropsychopharmacol. 50, 1119–1129 (2025). https://doi.org/10.1038/s41386-025-02051-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-025-02051-0