Abstract
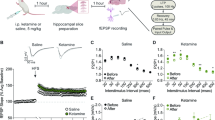
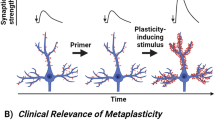
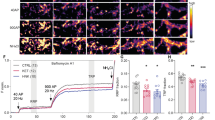
The pharmacologically active (R,S)-ketamine (ketamine) metabolite (2 R,6 R)-hydroxynorketamine (HNK) maintains ketamine’s preclinical antidepressant profile without adverse effects. While hypotheses have been proposed to explain how ketamine and its metabolites initiate their antidepressant-relevant effects, it remains unclear how sustained therapeutic actions arise following drug elimination. To distinguish the physiological mechanisms involved in the rapid from sustained actions of HNK, we utilized extracellular electrophysiology combined with pharmacology to develop an in vitro hippocampal slice incubation model that exhibited pharmacological fidelity to the 1) rapid synaptic potentiation induced by HNK at the Schaffer collateral-CA1 (SC-CA1) synapse during bath-application to slices collected from mice, and 2) maintenance of metaplastic (priming) activity that enhanced N-methyl-D-aspartate receptor (NMDAR) activation-dependent long-term potentiation (LTP) hours after in vivo dosing. We used this model to reveal novel mechanisms engaged in HNK’s temporally-sensitive antidepressant-relevant synaptic actions, finding that the induction of synaptic potentiation by HNK did not require NMDAR activity, but NMDAR activity was necessary to maintain synaptic priming. HNK required protein kinase A (PKA) activity to rapidly potentiate SC-CA1 neurotransmission to facilitate synaptic priming that persistently promoted LTP formation. HNK’s rapid actions were blocked by inhibitors of adenylyl cyclase 1 (AC1), but not an AC5 inhibitor. We conclude that HNK rapidly potentiates SC-CA1 synaptic efficacy, which then stimulates priming mechanisms that persistently favor plasticity. Targeting such priming mechanisms may be an effective antidepressant strategy, and our incubation model may aid in revealing novel pharmacological targets.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets produced during and/or analyzed for the present manuscript will be made available from the corresponding author upon reasonable request.
References
Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64.
Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013;74:250–56.
Fava M, Freeman MP, Flynn M, Judge H, Hoeppner BB, Cusin C, et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry. 2020;25:1592–603.
Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharm Rev. 2018;70:621–60.
Kim J-W, Suzuki K, Kavalali ET, Monteggia LM. Bridging rapid and sustained antidepressant effects of ketamine. Trends Mol Med. 2023;29:364–75.
Brown KA, Gould TD. Targeting metaplasticity mechanisms to promote sustained antidepressant actions. Mol Psychiatry. 2024:29:1114–27.
Krystal JH, Kaye AP, Jefferson S, Girgenti MJ, Wilkinson ST, Sanacora G, et al. Ketamine and the neurobiology of depression: Toward next-generation rapid-acting antidepressant treatments. Proc Natl Acad Sci USA. 2023;120:e2305772120.
Abdallah CG, De Feyter HM, Averill LA, Jiang L, Averill CL, Chowdhury GM, et al. The effects of ketamine on prefrontal glutamate neurotransmission in healthy and depressed subjects. Neuropsychopharmacology. 2018;43:2154–60.
Esterlis I, DellaGioia N, Pietrzak RH, Matuskey D, Nabulsi N, Abdallah CG, et al. Ketamine-induced reduction in mGluR5 availability is associated with an antidepressant response: an [11C] ABP688 and PET imaging study in depression. Mol Psychiatry. 2018;23:824–32.
Chowdhury GM, Zhang J, Thomas M, Banasr M, Ma X, Pittman B, et al. Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol Psychiatry. 2017;22:120–26.
Lorrain D, Baccei C, Bristow L, Anderson J, Varney M. Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience. 2003;117:697–706.
Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–27.
Zanos P, Gould T. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23:801–11.
Duman RS. Pathophysiology of depression and innovative treatments: remodeling glutamatergic synaptic connections. Dialogues Clin Neurosci. 2014;16:11–27.
Kavalali ET, Monteggia LM. Rapid homeostatic plasticity and neuropsychiatric therapeutics. Neuropsychopharmacology. 2023;48:54–60.
Chen M, Ma S, Liu H, Dong Y, Tang J, Ni Z, et al. Brain region–specific action of ketamine as a rapid antidepressant. Science. 2024;385:eado7010.
Ma S, Chen M, Jiang Y, Xiang X, Wang S, Wu Z, et al. Sustained antidepressant effect of ketamine through NMDAR trapping in the LHb. Nature. 2023;622:802–09.
Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature. 2018;554:317–22.
Gould TD, Zarate CA Jr., Thompson SM. Molecular Pharmacology and Neurobiology of Rapid-Acting Antidepressants. Annu Rev Pharm Toxicol. 2019;59:213–36.
Highland JN, Zanos P, Riggs LM, Georgiou P, Clark SM, Morris PJ, et al. Hydroxynorketamines: pharmacology and potential therapeutic applications. Pharm Rev. 2021;73:763–91.
Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–6.
Raja SM, Guptill JT, Mack M, Peterson M, Byard S, Twieg R, et al. A Phase 1 Assessment of the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of (2 R, 6 R)‐Hydroxynorketamine in Healthy Volunteers. Clin Pharmacol Ther. 2024;116:1314–24.
Zanos P, Highland JN, Stewart BW, Georgiou P, Jenne CE, Lovett J, et al. (2R, 6R)-hydroxynorketamine exerts mGlu2 receptor-dependent antidepressant actions. Proc Natl Acad Sci USA. 2019;116:6441–50.
Dutheil F, Dauchy S, Diry M, Sazdovitch V, Cloarec O, Mellottée L, et al. Xenobiotic-metabolizing enzymes and transporters in the normal human brain: regional and cellular mapping as a basis for putative roles in cerebral function. Drug Metab Disposition. 2009;37:1528–38.
Brown KA, Zanos P, Powels CF, Fix CJ, Michaelides M, Pereira EF, et al. Ketamine preservative benzethonium chloride potentiates hippocampal synaptic transmission and binds neurotransmitter receptors and transporters. Neuropharmacology. 2023;225:109403.
Riggs LM, Aracava Y, Zanos P, Fischell J, Albuquerque EX, Pereira EFR, et al. (2R,6R)-hydroxynorketamine rapidly potentiates hippocampal glutamatergic transmission through a synapse-specific presynaptic mechanism. Neuropsychopharmacology. 2020;45:426–36.
Riggs LM, Thompson SM, Gould TD. (2R, 6R)-hydroxynorketamine rapidly potentiates optically-evoked Schaffer collateral synaptic activity. Neuropharmacology. 2022:109153.
Georgiou P, Zanos P, Mou T-CM, An X, Gerhard DM, Dryanovski DI, et al. Experimenters’ sex modulates mouse behaviors and neural responses to ketamine via corticotropin releasing factor. Nat Neurosci. 2022;25:1191–200.
Brown KA, Carpenter JM, Preston CJ, Ludwig HD, Clay KB, Harn DA, et al. Lacto-N-fucopentaose-III ameliorates acute and persisting hippocampal synaptic plasticity and transmission deficits in a Gulf War Illness mouse model. Life Sci. 2021;279:119707.
Zanos P, Brown KA, Georgiou P, Yuan P, Zarate CA, Thompson SM, et al. NMDA Receptor Activation-Dependent Antidepressant-Relevant Behavioral and Synaptic Actions of Ketamine. J Neurosci. 2023;43:1038–50.
Thompson SM. Plasticity of synapses and reward circuit function in the genesis and treatment of depression. Neuropsychopharmacology. 2023;48:90–103.
Lumsden EW, Troppoli TA, Myers SJ, Zanos P, Aracava Y, Kehr J, et al. Antidepressant-relevant concentrations of the ketamine metabolite (2 R, 6 R)-hydroxynorketamine do not block NMDA receptor function. Proc Natl Acad Sci USA. 2019;116:5160–69.
Highland JN, Morris PJ, Konrath KM, Riggs LM, Hagen NR, Zanos P, et al. Hydroxynorketamine Pharmacokinetics and Antidepressant Behavioral Effects of (2, 6)-and (5 R)-Methyl-(2 R, 6 R)-hydroxynorketamines. ACS Chem Neurosci. 2022;13:510–23.
Zanos P, Highland JN, Liu X, Troppoli TA, Georgiou P, Lovett J, et al. (R)‐Ketamine exerts antidepressant actions partly via conversion to (2R, 6R)‐hydroxynorketamine, while causing adverse effects at sub‐anaesthetic doses. Br J Pharmacol. 2019;176:2573–92.
Highland JN, Morris PJ, Zanos P, Lovett J, Ghosh S, Wang AQ, et al. Mouse, rat, and dog bioavailability and mouse oral antidepressant efficacy of (2R, 6R)-hydroxynorketamine. J Psychopharmacol. 2019;33:12–24.
Riggs LM, Pereira EF, Thompson SM, Gould TD. cAMP-dependent protein kinase signaling is required for (2R, 6R)-hydroxynorketamine to potentiate hippocampal glutamatergic transmission. J Neurophysiol. 2024;131:64–74.
Trudeau L-E, Emery DG, Haydon PG. Direct modulation of the secretory machinery underlies PKA-dependent synaptic facilitation in hippocampal neurons. Neuron. 1996;17:789–97.
Kaneko M, Takahashi T. Presynaptic mechanism underlying cAMP-dependent synaptic potentiation. J Neurosci. 2004;24:5202–08.
Ostrom KF, LaVigne JE, Brust TF, Seifert R, Dessauer CW, Watts VJ, et al. Physiological roles of mammalian transmembrane adenylyl cyclase isoforms. Physiol Rev. 2022;102:815–57.
Conti AC, Maas JrJW, Muglia LM, Dave BA, Vogt SK, Tran TT, et al. Distinct regional and subcellular localization of adenylyl cyclases type 1 and 8 in mouse brain. Neuroscience. 2007;146:713–29.
Devasani K, Yao Y. Expression and functions of adenylyl cyclases in the CNS. Fluids Barriers CNS. 2022;19:23.
Brust TF, Alongkronrusmee D, Soto-Velasquez M, Baldwin TA, Ye Z, Dai M, et al. Identification of a selective small-molecule inhibitor of type 1 adenylyl cyclase activity with analgesic properties. Sci Signal. 2017;10:eaah5381.
Aleksandrova LR, Wang YT, Phillips AG. Ketamine and its metabolite,(2R, 6R)-HNK, restore hippocampal LTP and long-term spatial memory in the Wistar-Kyoto rat model of depression. Mol Brain. 2020;13:1–16.
Duman RS, Malberg J, Nakagawa S, D’Sa C. Neuronal plasticity and survival in mood disorders. Biol Psychiatry. 2000;48:732–9.
Parekh PK, Johnson SB, Liston C. Synaptic Mechanisms Regulating Mood State Transitions in Depression. Annu Rev Neurosci. 2022;45:581–601.
Kim JW, Monteggia LM. Increasing doses of ketamine curtail antidepressant responses and suppress associated synaptic signaling pathways. Behav Brain Res. 2020;380:112378.
Suzuki K, Kim J-W, Nosyreva E, Kavalali ET, Monteggia LM. Convergence of distinct signaling pathways on synaptic scaling to trigger rapid antidepressant action. Cell Rep. 2021;37:109918.
Izumi Y, Zorumski CF. Metaplastic effects of subanesthetic ketamine on CA1 hippocampal function. Neuropharmacology. 2014;86:273–81.
Graef JD, Newberry K, Newton A, Pieschl R, Shields E, Luan FN, et al. Effect of acute NR2B antagonist treatment on long-term potentiation in the rat hippocampus. Brain Res. 2015;1609:31–9.
Ribeiro PO, Tomé AR, Silva HB, Cunha RA, Antunes LM. Clinically relevant concentrations of ketamine mainly affect long-term potentiation rather than basal excitatory synaptic transmission and do not change paired-pulse facilitation in mouse hippocampal slices. Brain Res. 2014;1560:10–17.
Chen BK, Luna VM, LaGamma CT, Xu X, Deng SX, Suckow RF, et al. Sex-specific neurobiological actions of prophylactic (R,S)-ketamine, (2R,6R)-hydroxynorketamine, and (2S,6S)-hydroxynorketamine. Neuropsychopharmacology. 2020;45:1545–56.
Polis AJ, Fitzgerald PJ, Hale PJ, Watson BO. Rodent ketamine depression-related research: Finding patterns in a literature of variability. Behav Brain Res. 2019;376:112153.
Maxwell CR, Ehrlichman RS, Liang Y, Trief D, Kanes SJ, Karp J, et al. Ketamine produces lasting disruptions in encoding of sensory stimuli. J Pharm Exp Ther. 2006;316:315–24.
Brachman RA, McGowan JC, Perusini JN, Lim SC, Pham TH, Faye C, et al. Ketamine as a Prophylactic Against Stress-Induced Depressive-like Behavior. Biol Psychiatry. 2016;79:776–86.
Wang H, Pineda VV, Chan GC, Wong ST, Muglia LJ, Storm DR. Type 8 adenylyl cyclase is targeted to excitatory synapses and required for mossy fiber long-term potentiation. J Neurosci. 2003;23:9710–18.
Zhang M, Wang H. Ca2+-stimulated ADCY1 and ADCY8 regulate distinct aspects of synaptic and cognitive flexibility. Front Cell Neurosci. 2023;17:1215255.
Zhang M, Wang H. Mice overexpressing type 1 adenylyl cyclase show enhanced spatial memory flexibility in the absence of intact synaptic long-term depression. Learn Mem. 2013;20:352–57.
Wray NH, Schappi JM, Singh H, Senese NB, Rasenick MM. NMDAR-independent, cAMP-dependent antidepressant actions of ketamine. Mol Psychiatry. 2019;24:1833–43.
Chen J, Ding Q, An L, Wang H. Ca2+-stimulated adenylyl cyclases as therapeutic targets for psychiatric and neurodevelopmental disorders. Front Pharm. 2022;13:949384.
Kang H, Park P, Han M, Tidball P, Georgiou J, Bortolotto ZA, et al. (2 S, 6 S)-and (2 R, 6 R)-hydroxynorketamine inhibit the induction of NMDA receptor-dependent LTP at hippocampal CA1 synapses in mice. Brain and Neuroscience Advances. 2020;4:2398212820957847.
Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–63.
Lüscher C, Malenka RC. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb Perspect Biol. 2012;4:a005710.
Moaddel R, Abdrakhmanova G, Kozak J, Jozwiak K, Toll L, Jimenez L, et al. Sub-anesthetic concentrations of (R, S)-ketamine metabolites inhibit acetylcholine-evoked currents in α7 nicotinic acetylcholine receptors. Eur J Pharm. 2013;698:228–34.
Morris PJ, Moaddel R, Zanos P, Moore CE, Gould T, Zarate JrCA, et al. Synthesis and N-methyl-d-aspartate (NMDA) receptor activity of ketamine metabolites. Org Lett. 2017;19:4572–5.
Suzuki K, Nosyreva E, Hunt KW, Kavalali ET, Monteggia LM. Effects of a ketamine metabolite on synaptic NMDAR function. Nature. 2017;546:E1–3.
Barnes JR, Mukherjee B, Rogers BC, Nafar F, Gosse M, Parsons MP. The relationship between glutamate dynamics and activity-dependent synaptic plasticity. J Neurosci. 2020;40:2793–807.
Fukumoto K, Fogaça MV, Liu R-J, Duman C, Kato T, Li X-Y, et al. Activity-dependent brain-derived neurotrophic factor signaling is required for the antidepressant actions of (2 R, 6 R)-hydroxynorketamine. Proc Natl Acad Sci USA. 2019;116:297–302.
Cooper LN, Bear MF. The BCM theory of synapse modification at 30: interaction of theory with experiment. Nat Rev Neurosci. 2012;13:798–810.
Abraham WC, Mason-Parker SE, Bear MF, Webb S, Tate WP. Heterosynaptic metaplasticity in the hippocampus in vivo: a BCM-like modifiable threshold for LTP. Proc Natl Acad Sci. 2001;98:10924–29.
Buschler A, Manahan-Vaughan D. Brief environmental enrichment elicits metaplasticity of hippocampal synaptic potentiation in vivo. Front Behav Neurosci. 2012;6:85.
Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–30.
Huang Y-Y, Colino A, Selig DK, Malenka RC. The influence of prior synaptic activity on the induction of long-term potentiation. Science. 1992;255:730–33.
Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK, et al. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology. 2013;38:729–42.
Yang Y, Ju W, Zhang H, Sun L. Effect of ketamine on LTP and NMDAR EPSC in hippocampus of the chronic social defeat stress mice model of depression. Front Behav Neurosci. 2018;12:229.
Widman AJ, Stewart AE, Erb EM, Gardner E, McMahon LL. Intravascular ketamine increases theta-burst but not high frequency tetanus induced LTP at CA3-CA1 synapses within three hours and devoid of an increase in spine density. Front Synaptic Neurosci. 2018;10:8.
Kim J-W, Autry AE, Na ES, Adachi M, Björkholm C, Kavalali ET, et al. Sustained effects of rapidly acting antidepressants require BDNF-dependent MeCP2 phosphorylation. Nat Neurosci. 2021;24:1100–09.
Acknowledgements
We thank Patrick J. Morris and Craig J. Thomas (Division of Preclinical Innovation, National Center for Advancing Translational Sciences, NIH, Rockville, MD) for synthesizing and providing HNK.
Funding
Research was supported by NIH grant MH107615 and U.S. Department of Veterans Affairs Merit Awards 1I01BX006018 to TDG.
Author information
Authors and Affiliations
Contributions
KAB and TDG conceptualized the experiments conducted in the study. KAB and MIA completed the experiments and analyzed the data. KAB wrote the manuscript. All authors critically reviewed the manuscript and approved its final form.
Corresponding author
Ethics declarations
Competing interests
TDG is listed as co-author in patents and patent applications related to the pharmacology and use of (2R,6R)-HNK in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. TDG has assigned his patent rights to the University of Maryland, Baltimore, but will share a percentage of any royalties that may be received by the University of Maryland, Baltimore. The contents of this manuscript do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. TDG has served as a consultant for Boehringer Ingelheim during the preceding 3 years. All other authors report no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Brown, K.A., Ajibola, M.I. & Gould, T.D. Rapid hippocampal synaptic potentiation induced by ketamine metabolite (2R,6R)-hydroxynorketamine persistently primes synaptic plasticity. Neuropsychopharmacol. 50, 928–940 (2025). https://doi.org/10.1038/s41386-025-02085-4
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41386-025-02085-4