Abstract
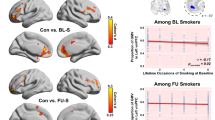
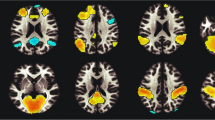
Despite the rise in electronic cigarette use in recent years, the neurobiological effects of daily e-cigarette use versus smoking cigarettes in young adults remains unknown. This study aimed to investigate the impact of regular, exclusive e-cigarette use on grey matter morphometry in young adults, age 18–25. Structural MRI data were collected from 3 distinct groups of participants (n = 78): daily, exclusive e-cigarette users; tobacco cigarette users; and non-using controls, to assess grey matter volume (GMV) differences. Voxel-based morphometry revealed significant GMV reductions in tobacco cigarette users in the left fusiform gyrus (FG), left and right inferior temporal gyrus (IFG), right middle temporal gyri, and right middle cingulate gyrus (MCG), compared to controls, as well as the anterior cingulate cortex (ACC), compared to both e-cigarette users and controls, even after adjusting for nicotine exposure history. Partial correlation analyses revealed that in tobacco cigarette users, GMV in the FG, ITG, MTG, and MCG displayed a strong, negative association with exposure history but not with nicotine dependence. GMV of the ACC was not associated with duration of use or nicotine dependence score, suggesting distinct relationships between ACC volume and smoking status and FG/ITG/MTG/MCG volume and smoking status. This indicates a distinct difference between regular tobacco cigarette and e-cigarette use, perhaps a relatively safer profile of e-cigarette use on GMV. These findings suggest that factors beyond nicotine, such as other toxicants in tobacco cigarette smoke, may contribute to the observed brain atrophy, or imply potential pre-existing vulnerabilities that might predispose individuals to take up smoking.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Data cannot be released because of privacy and ethics restrictions, but requests for access to de-identified patient-level data can be considered on reasonable request by contacting the corresponding author.
References
Hammond D, Rynard VL, Reid JL. Changes in prevalence of vaping among youths in the United States, Canada, and England from 2017 to 2019. JAMA Pediatr. 2020;174:797–9.
Government of Canada. Canadian Tobacco and Nicotine Survey (CTNS). Canada Stat. 2022;2021:1–5.
Kramarow EA, Elgaddal N. Current Electronic Cigarette Use Among Adults Aged 18 and Over: United States, 2021. NCHS Data Brief. 2023;475:1–8.
Johnson JM, Muilenburg JL, Rathbun SL, Yu X, Naeher LP, Wang JS. Elevated Nicotine Dependence Scores among Electronic Cigarette Users at an Electronic Cigarette Convention. J Community Health. 2018;43:164–74.
Jankowski M, Krzystanek M, Zejda JE, Majek P, Lubanski J, Lawson JA, et al. E-cigarettes are more addictive than traditional cigarettes—A study in highly educated young people. Int J Environ Res Public Health. 2019;16:4–13.
Chatterjee K, Alzghoul B, Innabi A, Meena N. Is vaping a gateway to smoking: A review of the longitudinal studies. Int J Adolesc Med Health. 2018;30:20160033.
Siddiqui F, Mishu M, Marshall AM, Siddiqi K. E-cigarette use and subsequent smoking in adolescents and young adults: a perspective. Expert Rev Respir Med. 2019;13:403–5.
Breslau N, Peterson EL. Smoking cessation in young adults: Age at initiation of cigarette smoking and other suspected influences. Am J Public Health. 1996;86:214–20.
Ali FRM, Agaku IT, Sharapova SR, Reimels EA, Homa DM. Onset of regular smoking before age 21 and subsequent nicotine dependence and cessation behavior among US adult smokers. Prev Chronic Dis. 2020;17:1–6.
Squeglia LM, Gray KM. Alcohol and Drug Use and the Developing Brain. Curr Psychiatry Rep. 2016;18. https://doi.org/10.1007/s11920-016-0689-y.
Arain M, Haque M, Johal L, Mathur P, Nel W, Rais A, et al. Maturation of the adolescent brain. Neuropsychiatr Dis Treat. 2013;9:449–61.
Peng P, Li M, Liu H, Tian YR, Chu SL, van Halm-Lutterodt N, et al. Brain structure alterations in respect to tobacco consumption and nicotine dependence: a comparative voxel-based morphometry study. Front Neuroanat. 2018;12. https://doi.org/10.3389/fnana.2018.00043.
Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Bierbrauer J, Kienast T, et al. Smoking and structural brain deficits: A volumetric MR investigation. Eur J Neurosci. 2006;24:1744–50.
Stoeckel LE, Chai XJ, Zhang J, Whitfield-Gabrieli S, Evins AE. Lower gray matter density and functional connectivity in the anterior insula in smokers compared with never smokers. Addiction Biol. 2016;21:972–81.
Chen Y, Chaudhary S, Wang W, Li CSR. Gray matter volumes of the insula and anterior cingulate cortex and their dysfunctional roles in cigarette smoking. Addiction Neurosci. 2022;1:100003.
Liao Y, Tang J, Liu T, Chen X, Hao W. Differences between smokers and non-smokers in regional gray matter volumes: A voxel-based morphometry study. Addiction Biology. 2012;17:977–80.
Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, et al. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry. 2004;55:77–84.
Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein EA. Factors underlying prefrontal and insula structural alterations in smokers. Neuroimage. 2011;54:42–48.
Fritz HC, Wittfeld K, Schmidt CO, Domin M, Grabe HJ, Hegenscheid K, et al. Current smoking and reduced gray matter volume - A voxel-based morphometry study. Neuropsychopharmacology. 2014;39:2594–2600.
Marques P, Piqueras L, Sanz MJ. An updated overview of e-cigarette impact on human health. Respir Res. 2021;22:1–14.
Goniewicz ML, Smith DM, Edwards KC, Blount BC, Caldwell KL, Feng J, et al. Comparison of Nicotine and Toxicant Exposure in Users of Electronic Cigarettes and Combustible Cigarettes. JAMA Netw Open. 2018;1:1–16.
Caliri AW, Tommasi S, Besaratinia A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat Res Rev Mutat Res. 2021;787:108365.
Durazzo TC, Meyerhoff DJ, Nixon SJ. Chronic Cigarette Smoking: Implications for Neurocognition and Brain Neurobiology. Int J Environ Res Public Health. 2010;7:3760–91.
Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K. The Fagerström Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27.
Rahman A, Mohamed MN, Jamshed S, Mahmood S, Baig MI. The development and assessment of modified Fagerstrom test for nicotine dependence scale among Malaysian single electronic cigarette users. J Pharm Bioallied Sci. 2020;12:671.
Rolls ET, Huang C-C, Lin C-P, Feng J, Joliot M. Automated anatomical labelling atlas 3. Neuroimage. 2020;206:116189.
Thomas Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65.
Saunders JB, Aasland OG, Babor TF, De La Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption‐II. Addiction. 1993;88:791–804.
Kunas SL, Hilbert K, Yang Y, Richter J, Hamm A, Wittmann A, et al. The modulating impact of cigarette smoking on brain structure in panic disorder: A voxel-based morphometry study. Soc Cogn Affect Neurosci. 2020;15:849–59.
Durazzo TC, Insel PS, Weiner MW. Greater regional brain atrophy rate in healthy elderly subjects with a history of cigarette smoking. Alzheimers Dementia. 2012;8:513–9.
Weidler C, Gramegna C, Müller D, Schrickel M, Habel U. Resting-state functional connectivity and structural differences between smokers and healthy non-smokers. Sci Rep. 2024;14:6878.
Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47.
Tranel D, Damasio H, Damasio AR. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 1997;35:1319–27.
Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat Neurosci. 1999;2:913–9.
Ishai A, Ungerleider LG, Martin A, Schouten JL, Haxby JV. Distributed representation of objects in the human ventral visual pathway. Proc Natl Acad Sci USA. 1999;96:9379–84.
Herath P, Kinomura S, Roland PE. Visual recognition: Evidence for two distinctive mechanisms from a PET study. Hum Brain Mapp. 2001;12:110–9.
Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–52.
Sergent J, Ohta S, Macdonald B. Functional Neuroanatomy of Face and Object Processing. Brain. 1992;115:15–36.
Wang X, Han Z, He Y, Caramazza A, Song L, Bi Y. Where color rests: Spontaneous brain activity of bilateral fusiform and lingual regions predicts object color knowledge performance. Neuroimage. 2013;76:252–63.
Weiner KS, Golarai G, Caspers J, Chuapoco MR, Mohlberg H, Zilles K, et al. The mid-fusiform sulcus: A landmark identifying both cytoarchitectonic and functional divisions of human ventral temporal cortex. Neuroimage. 2014;84:453–65.
Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–11.
Wilson SJ, Sayette MA, Delgado MR, Fiez JA. Instructed smoking expectancy modulates cue-elicited neural activity: A preliminary study. Nicotine Tobacco Res. 2005;7:637–45.
Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: Evidence from functional magnetic resonance imaging. Am J Psychiatry. 2002;159:954–60.
David S, Munafò M, Johansen-Berg H. Ventral striatum-nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers - fMRI. Soc Biological Psychiatry. 2005;58:488–94.
Zeng F, Hong W, Zha R, Li Y, Jin C, Liu Y, et al. Smoking related attention alteration in chronic obstructive pulmonary disease-smoking comorbidity. BMC Pulm Med. 2022;22:1–11.
Xue T, Dong F, Huang R, Tao Z, Tang J, Cheng Y, et al. Dynamic Neuroimaging Biomarkers of Smoking in Young Smokers. Front Psychiatry. 2020;11:663.
Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–52.
Janes AC, Farmer S, Peechatka AL, Frederick BDB, Lukas SE. Insula-dorsal anterior cingulate cortex coupling is associated with enhanced brain reactivity to smoking cues. Neuropsychopharmacology. 2015;40:1561–8.
Lerman C, Gu H, Loughead J, Ruparel K, Yang Y, Stein EA. Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry. 2014;71:523–30.
Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, et al. Neural substrates of smoking cue reactivity: A meta-analysis of fMRI studies. Neuroimage. 2012;60:252–62.
Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: A neurocognitive analysis. Nat Neurosci. 2004;7:211–4.
Lin X, Deng J, Shi L, Wang Q, Li P, Li H, et al. Neural substrates of smoking and reward cue reactivity in smokers: a meta-analysis of fMRI studies. Transl Psychiatry. 2020;10:97.
Allenby C, Falcone M, Wileyto EP, Cao W, Bernardo L, Ashare RL, et al. Neural cue reactivity during acute abstinence predicts short-term smoking relapse. Addiction Biol. 2020;25:1–9.
Chen WJA, Edwards RB, Romero RD, Parnell SE, Monk RJ. Long-term nicotine exposure reduces Purkinje cell number in the adult rat cerebellar vermis. Neurotoxicol Teratol. 2003;25:329–34.
Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacol Ther. 2009;122:125–39.
Trauth JA, Seidler FJ, Slotkin TA. An animal model of adolescent nicotine exposure: Effects on gene expression and macromolecular constituents in rat brain regions. Brain Res. 2000;867:29–39.
Akyol S, Erdogan S, Idiz N, Celik S, Kaya M, Ucar F, et al. The role of reactive oxygen species and oxidative stress in carbon monoxide toxicity: An in-depth analysis. Redox Report. 2014;19:180–9.
Hassan W, Noreen H, Rehman S, Kamal MA, da Rocha JBT. Association of Oxidative Stress with Neurological Disorders. Curr Neuropharmacol. 2021;20:1046–72.
Shen Z, Huang P, Qian W, Wang C, Yu H, Yang Y, et al. Severity of dependence modulates smokers’ functional connectivity in the reward circuit: a preliminary study. Psychopharmacology. 2016;233:2129–37.
Megías-Robles A, Cándido A, Maldonado A, Baltruschat S, Catena A. Differences between risk perception and risk-taking are related to impulsivity levels. Int J Clin Health Psychol. 2022;22:100318.
Kozak K, Lucatch AM, Lowe DJE, Balodis IM, MacKillop J, George TP. The neurobiology of impulsivity and substance use disorders: implications for treatment. Ann N Y Acad Sci. 2019;1451:71–91.
Manzione LC, Shan L, Azagba S. Associations Between Risk Perceptions and Cigarette, E-cigarette, and Dual-Product Use Among Canadian Adolescents. Tob Use Insights. 2020;13:1179173X2090378.
Government of Canada. Summary of results for the Canadian Student Tobacco, Alcohol and Drugs Survey 2018-19. Canada Statistics. 2019.
Funding
Kanwar Boparai is supported by funds from the Centre for Addiction and Mental Health, and The University of Toronto. Research was supported by grants to Laurie Zawertailo: Pfizer GRAND grant (WS2391913), CCS grant (707321-1) and CAMH Womenmind (1001079).
Author information
Authors and Affiliations
Contributions
Kanwar Boparai (Investigation, Formal analysis, Methodology, Writing—original draft, Writing—review & editing), Hsiang-Yuan Lin (Methodology, Writing—review & editing), Peter Selby (Conceptualization, Funding acquisition, Resources, Writing—review & editing), and Laurie Zawertailo (Conceptualization, Methodology, Funding acquisition, Resources, Supervision, Writing—review & editing).
Corresponding author
Ethics declarations
Competing interests
KB, HL and LZ have no competing interests to declare. PS holds the Vice-Chair, Research and Giblon Professor in Family Medicine Research, a university named Professorship at the University of Toronto.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boparai, K., Lin, HY., Selby, P. et al. Grey matter morphometry in young adult e-cigarette users, tobacco cigarette users & non-using controls. Neuropsychopharmacol. 50, 1455–1463 (2025). https://doi.org/10.1038/s41386-025-02086-3
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41386-025-02086-3