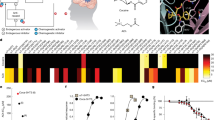
Abstract
Dopamine release in the nucleus accumbens core (NAcC) has long been associated with the promotion of motivated behavior. However, inhibited dopamine signaling can increase behavior in certain settings, such as during drug self-administration. While aversive environmental stimuli can reduce dopamine, it is unclear whether such stimuli reliably engage this mechanism in different contexts. Here we compared the physiological and behavioral responses to the same aversive stimulus in different designs to determine if there is uniformity in the manner that aversive stimuli are encoded and promote behavior. NAcC dopamine was measured using fiber photometry in male and female rats during cocaine self-administration sessions in which an acutely aversive 90 dB white noise was intermittently presented. In a separate group of rats, aversion-induced changes in dopamine were measured during an escape design in which operant responses terminated aversive white noise. Aversive white noise significantly reduced NAcC dopamine and increased cocaine self-administration in both male and female rats. The same relationship was observed in the escape design, in which white noise reduced dopamine and promoted the performance of escape behavior. In both designs, the magnitude of the dopamine reduction predicted behavioral performance. While prior research demonstrated that pharmacologically reduced dopamine signaling can promote intake, this report demonstrates that this physiological mechanism is naturally engaged by aversive environmental stimuli and is generalizable to non-drug contexts. These findings illustrate a common physiological signature in response to aversion that may promote both adaptive and maladaptive behavior.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl). 1999;142:343–51.
Robbins SJ, Ehrman RN, Childress AR, Cornish JW, O’Brien CP. Mood state and recent cocaine use are not associated with levels of cocaine cue reactivity. Drug Alcohol Depend. 2000;59:33–42.
Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl). 2003;170:62–72.
Fox HC, Hong K-IA, Siedlarz K, Sinha R. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2008;33:796–805.
Koob GF, Caine SB, Parsons L, Markou A, Weiss F. Opponent process model and psychostimulant addiction. Pharmacol Biochem Behav. 1997;57:513–21.
Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51.
Hammen C. Stress and depression. Annu Rev Clin Psychol. 2005;1:293–319.
Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97.
Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–94.
Blacktop JM, Seubert C, Baker DA, Ferda N, Lee G, Graf EN, et al. Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2. J Neurosci Off J Soc Neurosci. 2011;31:11396–403.
Twining RC, Wheeler DS, Ebben AL, Jacobsen AJ, Robble MA, Mantsch JR, et al. Aversive stimuli drive drug seeking in a state of low dopamine tone. Biol Psychiatry. 2015;77:895–902.
de Jong JW, Afjei SA, Pollak Dorocic I, Peck JR, Liu C, Kim CK, et al. A neural circuit mechanism for encoding aversive stimuli in the mesolimbic dopamine system. Neuron. 2019;101:133–151.e7.
Goedhoop JN, van den Boom BJ, Robke R, Veen F, Fellinger L, van Elzelingen W, et al. Nucleus accumbens dopamine tracks aversive stimulus duration and prediction but not value or prediction error. eLife. 2022;11:e82711.
Oleson EB, Gentry RN, Chioma VC, Cheer JF. Subsecond dopamine release in the nucleus accumbens predicts conditioned punishment and its successful avoidance. J Neurosci. 2012;32:14804–8.
van Elzelingen W, Goedhoop J, Warnaar P, Denys D, Arbab T, Willuhn I. A unidirectional but not uniform striatal landscape of dopamine signaling for motivational stimuli. Proc Natl Acad Sci. 2022;119:e2117270119.
Wheeler DS, Robble MA, Hebron EM, Dupont MJ, Ebben AL, Wheeler RA. Drug predictive cues activate aversion-sensitive striatal neurons that encode drug seeking. J Neurosci. 2015;35:7215–25.
De Wit H, Wise RA. Blockade of cocaine reinforcement in rats with the dopamine receptor blocker pimozide, but not with the noradrenergic blockers phentolamine or phenoxybenzamine. Can J Psychol Rev Can Psychol. 1977;31:195–203.
Yokel RA, Wise RA. Increased lever pressing for amphetamine after pimozide in rats: implications for a dopamine theory of reward. Science. 1975;187:547–9.
Yokel RA, Wise RA. Attenuation of intravenous amphetamine reinforcement by central dopamine blockade in rats. Psychopharmacology (Berl). 1976;48:311–8.
Suto N, Wise RA. Satiating effects of cocaine are controlled by dopamine actions in the nucleus accumbens core. J Neurosci. 2011;31:17917–22.
Suto N, Ecke LE, Wise RA. Control of within-binge cocaine-seeking by dopamine and glutamate in the core of nucleus accumbens. Psychopharmacology (Berl). 2009;205:431–9.
Harrison JM, Tracy WH. Use of auditory stimuli to maintain lever-pressing behavior. Science. 1955;121:373–4.
Barnes GW, Kish GB. Reinforcing properties of the termination of intense auditory stimulation. J Comp Physiol Psychol. 1957;50:40–43.
Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron. 2008;57:774–85.
Bakdash JZ, Marusich LR. Repeated measures correlation. Front Psychol. 2017;8:456.
Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–9.
Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl). 2000;152:140–8.
Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–31.
Caccamise A, Van Newenhizen E, Mantsch JR. Neurochemical mechanisms and neurocircuitry underlying the contribution of stress to cocaine seeking. J Neurochem. 2021;157:1697–713.
McReynolds JR, Wolf CP, Starck DM, Mathy JC, Schaps R, Krause LA, et al. Role of mesolimbic cannabinoid receptor 1 in stress-driven increases in cocaine self-administration in male rats. Neuropsychopharmacology. 2023;48:1–12.
Vranjkovic O, Gasser PJ, Gerndt CH, Baker DA, Mantsch JR. Stress-induced cocaine seeking requires a beta-2 adrenergic receptor-regulated pathway from the ventral bed nucleus of the stria terminalis that regulates CRF actions in the ventral tegmental area. J Neurosci Off J Soc Neurosci. 2014;34:12504–14.
Mantsch JR, Katz ES. Elevation of glucocorticoids is necessary but not sufficient for the escalation of cocaine self-administration by chronic electric footshock stress in rats. Neuropsychopharmacology. 2007;32:367–76.
Bachtell RK, Whisler K, Karanian D, Self DW. Effects of intranucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology (Berl). 2005;183:41–53.
Britton DR, Curzon P, Mackenzie RG, Kebabian JW, Williams JEG, Kerkman D. Evidence for involvement of both D1 and D2 receptors in maintaining cocaine self-administration. Pharmacol Biochem Behav. 1991;39:911–5.
Mahler SV, Brodnik ZD, Cox BM, Buchta WC, Bentzley BS, Quintanilla J, et al. Chemogenetic manipulations of ventral tegmental area dopamine neurons reveal multifaceted roles in cocaine abuse. J Neurosci. 2019;39:503–18.
Burgeno LM, Farero RD, Murray NL, Panayi MC, Steger JS, Soden ME, et al. Cocaine seeking and taking are oppositely regulated by dopamine. bioRxiv. 2023;04.09.536189; https://doi.org/10.1101/2023.04.09.536189.
Willuhn I, Burgeno LM, Groblewski PA, Phillips PEM. Excessive cocaine use results from decreased phasic dopamine signaling in the striatum. Nat Neurosci. 2014;17:704–9.
Kutlu MG, Zachry JE, Melugin PR, Cajigas SA, Chevee MF, Kelly SJ, et al. Dopamine release in the nucleus accumbens core signals perceived saliency. Curr Biol CB. 2021;31:4748–4761.e8.
Salinas-Hernández XI, Zafiri D, Sigurdsson T, Duvarci S. Functional architecture of dopamine neurons driving fear extinction learning. Neuron. 2023;111:3854–3870.e5.
Stelly CE, Haug GC, Fonzi KM, Garcia MA, Tritley SC, Magnon AP, et al. Pattern of dopamine signaling during aversive events predicts active avoidance learning. Proc Natl Acad Sci. 2019;116:13641–50.
Mohebi A, Pettibone JR, Hamid AA, Wong J-MT, Vinson LT, Patriarchi T, et al. Dissociable dopamine dynamics for learning and motivation. Nature. 2019;570:65–70.
Hart AS, Rutledge RB, Glimcher PW, Phillips PEM. Phasic dopamine release in the rat nucleus accumbens symmetrically encodes a reward prediction error term. J Neurosci. 2014;34:698–704.
Wenzel JM, Oleson EB, Gove WN, Cole AB, Gyawali U, Dantrassy HM, et al. Phasic dopamine signals in the nucleus accumbens that cause active avoidance require endocannabinoid mobilization in the midbrain. Curr Biol. 2018;28:1392–1404.e5.
Cui W, Aida T, Ito H, Kobayashi K, Wada Y, Kato S, et al. Dopaminergic signaling in the nucleus accumbens modulates stress-coping strategies during inescapable stress. J Neurosci Off J Soc Neurosci. 2020;40:7241–54.
Danjo T, Yoshimi K, Funabiki K, Yawata S, Nakanishi S. Aversive behavior induced by optogenetic inactivation of ventral tegmental area dopamine neurons is mediated by dopamine D2 receptors in the nucleus accumbens. Proc Natl Acad Sci USA. 2014;111:6455–60.
Dreyer JK, Herrik KF, Berg RW, Hounsgaard JD. Influence of phasic and tonic dopamine release on receptor activation. J Neurosci Off J Soc Neurosci. 2010;30:14273–83.
Lin Y-H, Yamahashi Y, Kuroda K, Faruk MO, Zhang X, Yamada K, et al. Accumbal D2R-medium spiny neurons regulate aversive behaviors through PKA-Rap1 pathway. Neurochem Int. 2021;143:104935.
Robble MA, Bozsik ME, Wheeler DS, Wheeler RA. Learned avoidance requires VTA KOR-mediated reductions in dopamine. Neuropharmacology. 2020;167:107996.
Zachry JE, Kutlu MG, Yoon HJ, Leonard MZ, Chevée M, Patel DD, et al. D1 and D2 medium spiny neurons in the nucleus accumbens core have distinct and valence-independent roles in learning. Neuron. 2023;112:835–49.
Diao Z, Yao L, Cheng Q, Wu M, Di Y, Qian Z, et al. Involvement of midbrain dopamine neuron activity in negative reinforcement learning in mice. Mol Neurobiol. 2021;58:5667–81.
Acknowledgements
The authors would like to thank Vaibhav Konanur, Matthew Gelin, Peter Lamberton, Kara Zimolzak and Mitch Roitman for technical assistance.
Funding
This work was supported by the Charles E. Kubly Mental Health Research Center and the National Institutes of Health (DA048280 to RAW, MCH, and JRM).
Author information
Authors and Affiliations
Contributions
EMG, DSW, JRM, MCH, and RAW designed the experiments. EMG, BEC, EG, LV, and DSW performed the surgeries, conducted the experiments, and analyzed the data. EMG, DSW, and RAW wrote the manuscript. BEC, JRM, MCH, LV, NP and DSW provided critical feedback on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Grafelman, E.M., Côté, B.E., Vlach, L. et al. Aversion-induced dopamine reductions predict drug-taking and escape behaviors. Neuropsychopharmacol. 50, 1376–1384 (2025). https://doi.org/10.1038/s41386-025-02101-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-025-02101-7