Abstract
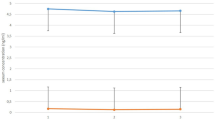
Anxiety disorders are prevalent psychiatric conditions that frequently emerge during adolescence. Among the neurobiological systems implicated in these disorders, the endocannabinoid (eCB) signaling system plays a crucial role, making it a promising target for therapeutic interventions. In addition to its direct effects on anxiety regulation, eCBs may also influence response to first-line pharmacologic treatments, such as selective serotonin reuptake inhibitors (SSRIs). However, little is known about developmental changes in eCB lipids—N-arachidonoylethanolamide (AEA) and 2-arachidonoylglycerol (2-AG)—or their relationship to anxiety symptoms and treatment response. Circulating AEA and 2-AG concentrations were measured in youth (aged 9–17, N = 199) with varying anxiety symptoms, assessed using the Screen for Child Anxiety-Related Disorders (SCARED). We evaluated how eCBs relate to developmental factors (e.g., demographics, biological variables) and anxiety symptoms (SCARED total). Additionally, we examined how eCB concentrations change in response to acute SSRI treatment in a subsample of adolescents (age 12–17, N = 41) with generalized anxiety disorder (GAD), who participated in an 8-week randomized placebo-controlled trial of escitalopram (15 mg/day, titrated to 20 mg/day). Body mass index (BMI) was positively correlated with circulating AEA, while 2-AG showed negative associations with age, female sex, and time-of-day. After adjusting for these variables, more severe anxiety symptoms were associated with higher AEA and lower 2-AG. Greater increases in 2-AG from baseline (without changes in AEA) were linked to improved treatment response in adolescents with GAD. Our study suggests that circulating eCBs may serve as biomarkers for anxiety severity and predictors of treatment response in youth.
ClinicalTrials.Gov Identifier: NCT02818751.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Research data are not shared.
References
Solmi M, Radua J, Olivola M, Croce E, Soardo L, Salazar de Pablo G, et al. Age at onset of mental disorders worldwide: large-scale meta-analysis of 192 epidemiological studies. Mol Psychiatry. 2021;27:281–95.
Essau CA, Lewinsohn PM, Olaya B, Seeley JR. Anxiety disorders in adolescents and psychosocial outcomes at age 30. J Affect Disord. 2014;163:125–32.
Ginsburg GS, Becker-Haimes EM, Keeton C, Kendall PC, Iyengar S, Sakolsky D, et al. Results from the child/adolescent anxiety multimodal extended long-term study (CAMELS): primary anxiety outcomes. J Am Acad Child Adolesc Psychiatry. 2018. https://doi.org/10.1016/j.jaac.2018.03.017.
Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. 2005;62:593.
Warner EN, Ammerman RT, Glauser TA, Pestian JP, Agasthya G, Strawn JR. Developmental epidemiology of pediatric anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2023;32:511–30.
Beesdo K, Pine DS, Lieb R, Wittchen H-U. Incidence and risk patterns of anxiety and depressive disorders and categorization of generalized anxiety disorder. Arch Gen Psychiatry. 2010;67:47–57.
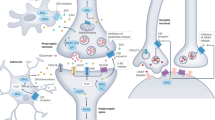
Lutz B, Marsicano G, Maldonado R, Hillard CJ. The endocannabinoid system in guarding against fear, anxiety and stress. Nat Rev Neurosci. 2015;16:705–18.
Gunduz-Cinar O, Hill MN, McEwen BS, Holmes A. Amygdala FAAH and anandamide: Mediating protection and recovery from stress. Trends Pharm Sci. 2013;34:637–44.
Baumel WT, Strawn JR. Neurobiology of treatment in pediatric anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2023;32:589–600.
Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–4.
Hillard CJ. The endocannabinoid signaling system in the CNS: a primer. Int Rev Neurobiol. 2015;125:1–47.
deRoon-Cassini TA, Stollenwerk TM, Beatka M, Hillard CJ. Meet your stress management professionals: the endocannabinoids. Trends Mol Med. 2020;26:953–68.
Umathe SN, Manna SSS, Jain NS. Involvement of endocannabinoids in antidepressant and anti-compulsive effect of fluoxetine in mice. Behav Brain Res. 2011;223:125–34.
Hill MN, Carrier EJ, McLaughlin RJ, Morrish AC, Meier SE, Hillard CJ, et al. Regional alterations in the endocannabinoid system in an animal model of depression: effects of concurrent antidepressant treatment. J Neurochem. 2008;106:2322–36.
Hillard CJ. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology. 2018;43:155–72.
Gowatch LC, Evanski JM, Ely SL, Zundel CG, Bhogal A, Carpenter C, et al. Endocannabinoids and stress-related neurospsychiatric disorders: a systematic review and meta-analysis of basal concentrations and response to acute psychosocial stress. Cannabis Cannabinoid Res. (2024). https://doi.org/10.1089/can.2023.0246.
Pharmaceuticals J Jazz Pharmaceuticals Provides Update on Phase 2 Trial of Investigational JZP150 in Adult Patients with Post-Traumatic Stress Disorder\. (2023).
Mayo LM, Asratian A, Lindé J, Morena M, Haataja R, Hammar V, et al. Elevated anandamide, enhanced recall of fear extinction, and attenuated stress responses following inhibition of fatty acid amide hydrolase: a randomized, controlled experimental medicine trial. Biol Psychiatry. 2020. https://doi.org/10.1016/j.biopsych.2019.07.034.
Mayo LM, Asratian A, Lindé J, Holm L, Nätt D, Augier G, et al. Protective effects of elevated anandamide on stress and fear-related behaviors: translational evidence from humans and mice. Mol Psychiatry. 2020. https://doi.org/10.1038/s41380-018-0215-1.
Mayo LM, Rabinak CA, Hill MN, Heilig M. Targeting the endocannabinoid system in the treatment of posttraumatic stress disorder: a promising case of preclinical-clinical translation? Biol Psychiatry. 2022;91:262–72.
Sharafi A, Pakkhesal S, Fakhari A, Khajehnasiri N, Ahmadalipour A. Rapid treatments for depression: endocannabinoid system as a therapeutic target. Neurosci Biobehav Rev. 2022;137:104635.
Desai S, Borg B, Cuttler C, Crombie K, Rabinak C, Hill M, et al. A systematic review and meta-analysis on the effects of exercise on the endocannabinoid system. Cannabis Cannabinoid Res. 2021;7:388–408.
Marusak HA, Ely SL, Zundel CG, Gowatch LC, Shampine M, Carpenter C, et al. Endocannabinoid dysregulation and PTSD in urban adolescents: associations with anandamide concentrations and FAAH genotype. Psychopharmacology. 2024. https://doi.org/10.1007/s00213-024-06717-3.
Strawn JR, Lu L, Peris TS, Levine A, Walkup JT. Research review: pediatric anxiety disorders—What have we learnt in the last 10 years? J Child Psychol Psychiatry. 2021;62:114–39.
Ryan ND. Treatment of anxiety disorders in youths: filling the cup further. Am J Psychiatry. 2017;174:723–4.
Meyer HC, Lee FS, Gee DG. The role of the endocannabinoid system and genetic variation in adolescent brain development. Neuropsychopharmacology. 2018;43:21–33.
Marusak HA. The role of cannabinoids in shaping lifespan neurodevelopment. J Neurosci Res. 2022;100:709–12.
Strawn JR, Mills JA, Schroeder H, Mossman SA, Varney ST, Ramsey LB, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81:20m13396.
Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M. Psychometric properties of the screen for child anxiety related emotional disorders (SCARED): a replication study. J Am Acad Child Adolesc Psychiatry. 1999. https://doi.org/10.1097/00004583-199910000-00011.
Albano AM, Silverman WK. Anxiety disorders interview schedule for DSM-IV: Clinician manual. (No Title). (1996). 1996.
The Pediatric Anxiety Rating Scale (PARS): development and psychometric properties. J Am Acad Child Adolesc Psychiatry. (2002);41:1061-9.
Guy W. ECDEU assessment manual for psychopharmacology. US Department of Health, Education, and Welfare, Public Health Service; (1976).
Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry. 2007;4:28–37.
Pastor A, Farré M, Fitó M, Fernandez-Aranda F, de la Torre R. Analysis of ECs and related compounds in plasma: artifactual isomerization and ex vivo enzymatic generation of 2-MGs[S]. J Lipid Res. 2014;55:966–77.
Qi M, Morena M, Vecchiarelli HA, Hill MN, Schriemer DC. A robust capillary liquid chromatography/tandem mass spectrometry method for quantitation of neuromodulatory endocannabinoids. Rapid Commun Mass Spectrom. 2015. https://doi.org/10.1002/rcm.7277.
Hill MN, Bierer LM, Makotkine I, Golier JA, Galea S, McEwen BS, et al. Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the world trade center attacks. Psychoneuroendocrinology. 2013;38:2952–61.
Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. Introd Mediat Moderat Cond Process Anal A Regres-Based Approach 2013;xvii:507.
Strawn JR, Geracioti L, Rajdev N, Clemenza K, Levine A. Pharmacotherapy for generalized anxiety disorder in adult and pediatric patients: an evidence-based treatment review. Expert Opin Pharmacother. 2018;19:1057–70.
Varigonda AL, Jakubovski E, Taylor MJ, Freemantle N, Coughlin C, Bloch MH. Systematic review and meta-analysis: early treatment responses of selective serotonin reuptake inhibitors in pediatric major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2015;54:557–64.
Mayo LM, Rabinak CA, Hill MN, Heilig M. Targeting the endocannabinoid system in the treatment of PTSD: a promising case of preclinical-clinical translation? Biol Psychiatry. 2021. https://doi.org/10.1016/j.biopsych.2021.07.019.
Aguilera Vasquez N, Nielsen DE. The endocannabinoid system and eating behaviours: a review of the current state of the evidence. Curr Nutr Rep. 2022;11:665–74.
Amir Hamzah K, Toms LM, Kucharski N, Orr J, Turner NP, Hobson P, et al. Sex-dimorphism in human serum endocannabinoid and n-acyl ethanolamine concentrations across the lifespan. Sci Rep. 2023;13:1–11.
Hanlon EC, Tasali E, Leproult R, Stuhr KL, Doncheck E, de Wit H, et al. Circadian rhythm of circulating levels of the endocannabinoid 2-arachidonoylglycerol. J Clin Endocrinol Metab. 2015;100:220–6.
Hill MN, Miller GE, Carrier EJ, Gorzalka BB, Hillard CJ. Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology. 2009. https://doi.org/10.1016/j.psyneuen.2009.03.013.
Petrowski K, Kirschbaum C, Gao W, Hardt J, Conrad R. Blood endocannabinoid levels in patients with panic disorder. Psychoneuroendocrinology. 2020;122:104905.
Hauer D, Schelling G, Gola H, Campolongo P, Morath J, Roozendaal B, et al. Plasma concentrations of endocannabinoids and related primary fatty acid amides in patients with post-traumatic stress disorder. PLoS One. 2013;8:e62741.
Morena M, Patel S, Bains JS, Hill MN. Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology. 2016;41:80–102.
Hill MN, McEwen BS. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:791–7.
Silveira KM, Wegener G, Joca SRL. Targeting 2-arachidonoylglycerol signalling in the neurobiology and treatment of depression. Basic Clin Pharm Toxicol. 2021;129:3–14.
Hasbi A, Madras BK, George SR. Endocannabinoid system and exogenous cannabinoids in depression and anxiety: a review. Brain Sci. 2023;13:325.
Bedse G, Hill MN, Patel S. 2-arachidonoylglycerol modulation of anxiety and stress adaptation: from grass roots to novel therapeutics. Biol Psychiatry. 2020;88:520–30.
Bluett RJ, Báldi R, Haymer A, Gaulden AD, Hartley ND, Parrish WP, et al. Endocannabinoid signalling modulates susceptibility to traumatic stress exposure. Nat Commun. 2017;8:14782.
Baumel WT, Lu L, Huang X, Drysdale AT, Sweeny JA, Gong Q, et al. Neurocircuitry of treatment in anxiety disorders. Biomarkers Neuropsychiatry. 2022;6:100052.
Chester LA, Englund A, Chesney E, Oliver D, Wilson J, Sovi S, et al. Effects of cannabidiol and delta-9-tetrahydrocannabinol on plasma endocannabinoid levels in healthy volunteers: a randomized double-blind four-arm crossover study. Cannabis Cannabinoid Res. 2024;9:188–98.
Sloan ME, Grant CW, Stangl BL, Klepp TD, Brewton HW, Cinar R, et al. The effects of acute alcohol administration on circulating endocannabinoid levels in humans. Addict Biol. 2022;27:e13197.
Gamaleddin IH, Trigo JM, Gueye AB, Zvonok A, Makriyannis A, Goldberg SR, et al. Role of the endogenous cannabinoid system in nicotine addiction: Novel insights. Front Psychiatry. 2015;6:1–12.
Acknowledgements
The authors would like to thank the following individuals and organizations for their assistance with study design, recruitment, data collection, and/or analysis: Amanpreet Bhogal, Shelley Paulisin, Jovan Jande, Emily Crisan, Alexander Jakubiec, Shravya Chanamolu, Sneha Bhargava, Myles Davis, Zazai Owens, Carla Hannah, Iveta Kopil, Shreya Desai, Cameron Martella, Autumm Heeter, Julia Evanski, Ahmad Almaat, Dr. Christine Rabinak, Leah Gowatch, Dr. Leslie Lundahl, Dr. Krishna Rao Maddipati, Dr. Laura Benjamins, Dr. Sharon Marshall, Dr. Christopher Youngman, Wayne Pediatrics, The Children’s Center, The Wayne State University Lipidomics Core and MR Research Facility. A generative large language AI model was used to assist with copy editing the manuscript.
Funding
This study was supported by National Institutes of Health Award K01MH119241 (to HM), a Richard Barber Interdisciplinary Research Grant (to HM and JB), and a Social & Behavioral Determinants of Health Research Stimulus Program Pilot Project Grant from the Wayne State University Office of the Provost (to HM and JB). Dr. Strawn was also supported in part by the Yung Family Foundation and the National Institute of Child Health & Development (R01HD099775 and R01HD098757) and the clinical trial was funded by the National institute of Mental Health (K23MH106037). Dr. Marusak was partially supported by R01MH132830 and R21HD105882. Dr. Zundel was partially supported by F32 MH133274, and Ms. Ely was partially supported by T32GM139807 and a Merrill Palmer Skillman Institute for Child and Family Development Fellowship. This study was partly supported by grants from the National Institutes of Health S10RR027926 and S10OD032292 to the Lipidomics Core Facility of Wayne State University.
Author information
Authors and Affiliations
Contributions
Hilary A. Marusak: Substantial contributions to the conception and design of the work; acquisition, analysis, and interpretation of data; drafting the manuscript and revising it critically for important intellectual content; final approval of the version to be published; accountable for all aspects of the work, ensuring the accuracy and integrity of the work. Clara G. Zundel: Substantial contributions to the acquisition of data; revising the manuscript critically for important intellectual content; final approval of the version to be published; agreement to be accountable for the accuracy and integrity of the work. Tehmina Shakir: Revising the manuscript critically for important intellectual content; final approval of the version to be published; agreement to be accountable for the accuracy and integrity of the work. Samantha L. Ely: Substantial contributions to the acquisition of data; revising the manuscript critically for important intellectual content; final approval of the version to be published; agreement to be accountable for the accuracy and integrity of the work. Carmen Carpenter: Substantial contributions to the acquisition of data; revising the manuscript critically for important intellectual content; final approval of the version to be published; agreement to be accountable for the accuracy and integrity of the work. MacKenna Shampine: Substantial contributions to the acquisition of data; revising the manuscript critically for important intellectual content; final approval of the version to be published; agreement to be accountable for the accuracy and integrity of the work. Reem Tamimi: Revising the manuscript critically for important intellectual content; final approval of the version to be published; agreement to be accountable for the accuracy and integrity of the work. Mariya Matsko: Substantial contributions to the acquisition of data; revising the manuscript critically for important intellectual content; final approval of the version to be published; agreement to be accountable for the accuracy and integrity of the work. Sarah Rogers: Substantial contributions to the acquisition of data; revising the manuscript critically for important intellectual content; final approval of the version to be published; agreement to be accountable for the accuracy and integrity of the work. Jennifer Losiowski: Revising the manuscript critically for important intellectual content; final approval of the version to be published; agreement to be accountable for the accuracy and integrity of the work. Emilie O’Mara: Revising the manuscript critically for important intellectual content; final approval of the version to be published; agreement to be accountable for the accuracy and integrity of the work. Alaina M. Jaster: Revising the manuscript critically for important intellectual content; final approval of the version to be published; agreement to be accountable for the accuracy and integrity of the work. Kamakashi Sharma: Revising the manuscript critically for important intellectual content; final approval of the version to be published; agreement to be accountable for the accuracy and integrity of the work. Terri A. deRoon-Cassini: Revising the manuscript critically for important intellectual content; final approval of the version to be published; agreement to be accountable for the accuracy and integrity of the work. Cecilia J. Hillard: Revising the manuscript critically for important intellectual content; final approval of the version to be published; agreement to be accountable for the accuracy and integrity of the work. Heidi K. Schroeder: Substantial contributions to the acquisition of data; revising the manuscript critically for important intellectual content; final approval of the version to be published; agreement to be accountable for the accuracy and integrity of the work. Jeffrey A. Mills: Substantial contributions to the analysis and interpretation of data; revising the manuscript critically for important intellectual content; final approval of the version to be published; agreement to be accountable for the accuracy and integrity of the work. Jeffrey R. Strawn: Substantial contributions to the conception and design of the work; acquisition and analysis of data; revising the manuscript critically for important intellectual content; final approval of the version to be published; accountable for ensuring the accuracy and integrity of the work. Jeanne Barcelona: Substantial contributions to the conception and design of the work; acquisition and analysis of data; revising the manuscript critically for important intellectual content; final approval of the version to be published; accountable for ensuring the accuracy and integrity of the work.
Corresponding author
Ethics declarations
Competing interests
Dr. Hillard is a member of the Scientific Advisory Board and has equity in Formulate Biosciences. Dr. Strawn has received research support from the National Institutes of Health (NIMH/NIEHS/NICHD) and material support from Myriad Genetics. He receives royalties from Springer and Cambridge University Press, honoraria from the Neuroscience Education Institute, and serves as an author for UpToDate. He has consulted to MindMed, Abbvie (Cerevel), Otsuka, Vistagen, Supernus and Genomind. Drs. Strawn and Mills receive research support from the Yung Family Foundation. All other authors report no biomedical financial interests or potential conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marusak, H.A., Zundel, C.G., Shakir, T. et al. Circulating endocannabinoids in children and adolescents: associations with anxiety and the impact of selective serotonin reuptake inhibitors. Neuropsychopharmacol. 50, 1606–1614 (2025). https://doi.org/10.1038/s41386-025-02155-7
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41386-025-02155-7