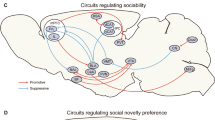
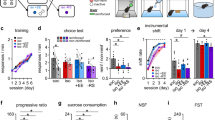
Abstract
Social deficits are a core feature of many psychiatric disorders. Importantly, aberrant social experiences during childhood and adolescence profoundly influence maturation of the brain function and induce social impairments in adulthood. Social isolation, especially among youth, has been highlighted as a serious risk to mental health. As a result, understanding the consequences of social isolation on a mechanistic level has become increasingly urgent. Recent rodent studies have revealed that social isolation during development induces widespread changes in adult brain structures, particularly in the prefrontal cortical circuits, and causes altered social behavior. These findings led us to develop two models proposing that social isolation may cause deficits through either concurrent (social deprivation model) or subsequent (developmental mismatch model) to social disruption. Building on these two models, this review examines how these models provide complementary perspectives on the influence of social isolation on the progression of schizophrenia, from the prodromal to the psychotic phase, a condition in which genetic and environmental risk factors are closely linked to social isolation. Advancing our understanding of treatment timing and targets for isolation-induced social deficits through the lenses of these models may help identify the optimal developmental timing for effective interventions and prevention strategies for schizophrenia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cuthbert BN, Workgroup NR. The RDoC framework: continuing commentary. World Psychiatry. 2014;13:196–7.
Bicks LK, Koike H, Akbarian S, Morishita H. Prefrontal cortex and social cognition in mouse and man. Front Psychol. 2015;6:1805.
Segrin C. Social skills deficits associated with depression. Clin. Psychol. Rev. 2000;20:379–403.
Champagne FA, Curley JP. How social experiences influence the brain. Curr. Opin. Neurobiol. 2005;15:704–9.
Sachser N, Kaiser S, Hennessy MB. Behavioural profiles are shaped by social experience: when, how and why. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013;368:20120344.
Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence?. Nat. Rev. Neurosci. 2008;9:947–57.
Mushtaq R, Shoib S, Shah T, Mushtaq S. Relationship between loneliness, psychiatric disorders and physical health ? A review on the psychological aspects of loneliness. J. Clin. Diagn. Res. 2014;8:WE01–04.
Brandt L, Liu S, Heim C, Heinz A. The effects of social isolation stress and discrimination on mental health. Transl. Psychiatry. 2022;12:398.
Qualter P, Brown SL, Rotenberg KJ, Vanhalst J, Harris RA, Goossens L, et al. Trajectories of loneliness during childhood and adolescence: predictors and health outcomes. J. Adolesc. 2013;36:1283–93.
Erickson DH, Beiser M, Iacono WG, Fleming JA, Lin TY. The role of social relationships in the course of first-episode schizophrenia and affective psychosis. Am. J. Psychiatry. 1989;146:1456–61.
Parnas J, Bovet P, Zahavi D. Schizophrenic autism: clinical phenomenology and pathogenetic implications. World Psychiatry. 2002;1:131–6.
Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388:86–97.
Reh RK, Dias BG, Nelson CA, Kaufer D, Werker JF, Kolb B, et al. Critical period regulation across multiple timescales. Proc. Natl. Acad. Sci. USA. 2020;117:23242–51.
Hensch TK. Critical period regulation. Annu Rev. Neurosci. 2004;27:549–79.
Freedman DG, King JA, Elliot O. Critical period in the social development of dogs. Science. 1961;133:1016–7.
Harlow HF, Novak MA. Psychopathological perspectives. Perspect. Biol. Med. 1973;16:461–78.
Burke AR, McCormick CM, Pellis SM, Lukkes JL. Impact of adolescent social experiences on behavior and neural circuits implicated in mental illnesses. Neurosci. Biobehav Rev. 2017;76:280–300.
Palagi E, Burghardt GM, Smuts B, Cordoni G, Dall'Olio S, Fouts HN, et al. Rough-and-tumble play as a window on animal communication. Biol. Rev. Camb. Philos. Soc. 2016;91:311–27.
Pellis SM, Pasztor TJ. The developmental onset of a rudimentary form of play fighting in C57 mice. Dev. Psychobiol. 1999;34:175–82.
Arakawa H. Ethological approach to social isolation effects in behavioral studies of laboratory rodents. Behav. Brain Res. 2018;341:98–108.
Bicks LK, Yamamuro K, Flanigan ME, Kim JM, Kato D, Lucas EK, et al. Prefrontal parvalbumin interneurons require juvenile social experience to establish adult social behavior. Nat. Commun. 2020;11:1003.
Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337:1357–60.
Yamamuro K, Bicks LK, Leventhal MB, Kato D, Im S, Flanigan ME, et al. A prefrontal-paraventricular thalamus circuit requires juvenile social experience to regulate adult sociability in mice. Nat. Neurosci. 2020;23:1240–52.
S Musardo, A Contestabile, M Knoop, O Baud, C Bellone, Oxytocin neurons mediate the effect of social isolation via the VTA circuits. Elife. 2022;11:e73421.
Bator E, Latusz J, Głowacka U, Radaszkiewicz A, Mudlaff K, Maćkowiak M. Adolescent social isolation affects schizophrenia-like behavior in the MAM-E17 model of schizophrenia. Neurotox. Res. 2018;34:305–23.
Hol T, Van den Berg CL, Van Ree JM, Spruijt BM. Isolation during the play period in infancy decreases adult social interactions in rats. Behav. Brain Res. 1999;100:91–7.
Zhang X, Xun Y, Wang L, Zhang J, Hou W, Ma H, et al. Involvement of the dopamine system in the effect of chronic social isolation during adolescence on social behaviors in male C57 mice. Brain Res. 2021;1765:147497.
Fone KC, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci. Biobehav Rev. 2008;32:1087–102.
Li DC, Hinton EA, Gourley SL. Persistent behavioral and neurobiological consequences of social isolation during adolescence. Semin Cell Dev. Biol. 2021;118:73–82.
Mack NR, Bouras NN, Gao WJ. Prefrontal regulation of social behavior and related deficits: insights from rodent studies. Biol. Psychiatry. 2024;96:85–94.
Walker DM, Cunningham AM, Gregory JK, Nestler EJ. Long-term behavioral effects of post-weaning social isolation in males and females. Front Behav. Neurosci. 2019;13:66.
Xiong Y, Hong H, Liu C, Zhang YQ. Social isolation and the brain: effects and mechanisms. Mol. Psychiatry. 2023;28:191–201.
Zhang XQ, Jiang HJ, Xu L, Yang SY, Wang GZ, Jiang HD, et al. The metabotropic glutamate receptor 2/3 antagonist LY341495 improves working memory in adult mice following juvenile social isolation. Neuropharmacology. 2020;177:108231.
Leussis MP, Lawson K, Stone K, Andersen SL. The enduring effects of an adolescent social stressor on synaptic density, part II: Poststress reversal of synaptic loss in the cortex by adinazolam and MK-801. Synapse. 2008;62:185–92.
Kim GS, Lee H, Jeong Y. Altered dorsal functional connectivity after post-weaning social isolation and resocialization in mice. Neuroimage. 2021;245:118740.
Yamamuro K, Yoshino H, Ogawa Y, Makinodan M, Toritsuka M, Yamashita M, et al. Social isolation during the critical period reduces synaptic and intrinsic excitability of a subtype of pyramidal cell in mouse prefrontal cortex. Cereb. Cortex. 2018;28:998–1010.
Li X, Sun H, Zhu Y, Wang F, Wang X, Han L, et al. Dysregulation of prefrontal parvalbumin interneurons leads to adult aggression induced by social isolation stress during adolescence. Front Mol. Neurosci. 2022;15:1010152.
Makinodan M, Ikawa D, Yamamuro K, Yamashita Y, Toritsuka M, Kimoto S, et al. Effects of the mode of re-socialization after juvenile social isolation on medial prefrontal cortex myelination and function. Sci. Rep. 2017;7:5481.
Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J. Comp. Neurol. 2005;492:145–77.
Penzo MA, Gao C. The paraventricular nucleus of the thalamus: an integrative node underlying homeostatic behavior. Trends Neurosci. 2021;44:538–49.
Hung LW, Neuner S, Polepalli JS, Beier KT, Wright M, Walsh JJ, et al. Gating of social reward by oxytocin in the ventral tegmental area. Science. 2017;357:1406–11.
Huang WC, Zucca A, Levy J, Page DT. Social Behavior is modulated by valence-encoding mPFC-Amygdala sub-circuitry. Cell Rep. 2020;32:107899.
Resendez SL, Namboodiri V, Otis JM, Eckman L, Rodriguez-Romaguera J, Ung RL, et al. Social stimuli induce activation of oxytocin neurons within the paraventricular nucleus of the hypothalamus to promote social behavior in male mice. J. Neurosci. 2020;40:2282–95.
Paine TA, Swedlow N, Swetschinski L. Decreasing GABA function within the medial prefrontal cortex or basolateral amygdala decreases sociability. Behav. Brain Res. 2017;317:542–52.
Zhang XQ, Yu ZP, Ling Y, Zhao QQ, Zhang ZY, Wang ZC, Shen HW. Enduring effects of juvenile social isolation on physiological properties of medium spiny neurons in nucleus accumbens. Psychopharmacology. 2019;236:3281–9.
Lukkes JL, Mokin MV, Scholl JL, Forster GL. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm. Behav. 2009;55:248–56.
Shan Q, Hu Y, Chen S, Tian Y. Nucleus accumbens dichotomically controls social dominance in male mice. Neuropsychopharmacology. 2022;47:776–87.
Baez-Mendoza R, Schultz W. The role of the striatum in social behavior. Front Neurosci. 2013;7:233.
Begni V, Zampar S, Longo L, Riva MA. Sex differences in the enduring effects of social deprivation during adolescence in rats: implications for psychiatric disorders. Neuroscience. 2020;437:11–22.
Felix-Ortiz AC, Tye KM. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J. Neurosci. 2014;34:586–95.
ML Phillips, HA Robinson, L Pozzo-Miller, Ventral hippocampal projections to the medial prefrontal cortex regulate social memory. Elife. 2019;8:e44182.
Harte MK, Powell SB, Swerdlow NR, Geyer MA, Reynolds GP. Deficits in parvalbumin and calbindin immunoreactive cells in the hippocampus of isolation reared rats. J. Neural Transm. 2007;114:893–8.
Chini M, Hanganu-Opatz IL. Prefrontal cortex development in health and disease: lessons from rodents and humans. Trends Neurosci. 2021;44:227–40.
CB Klune, B Jin, LA DeNardo, Linking mPFC circuit maturation to the developmental regulation of emotional memory and cognitive flexibility. Elife. 2021;10:e64567.
Ueda S, Niwa M, Hioki H, Sohn J, Kaneko T, Sawa A, Sakurai T. Sequence of molecular events during the maturation of the developing mouse prefrontal cortex. Mol. Neuropsychiatry. 2015;1:94–104.
Niwa M, Kamiya A, Murai R, Kubo K, Gruber AJ, Tomita K, et al. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron. 2010;65:480–9.
Leventhal MB, Morishita H. How childhood social isolation causes social dysfunction: deprivation or mismatch?. Trends Cogn. Sci. 2024;28:699–701.
Lee CR, Chen A, Tye KM. The neural circuitry of social homeostasis: Consequences of acute versus chronic social isolation. Cell. 2021;184:2794–5.
Wiesel TN. Postnatal development of the visual cortex and the influence of environment. Nature. 1982;299:583–91.
Hodges TE, Green MR, Simone JJ, McCormick CM. Effects of social context on endocrine function and Zif268 expression in response to an acute stressor in adolescent and adult rats. Int J. Dev. Neurosci. 2014;35:25–34.
Hodges TE, McCormick CM. Adolescent and adult male rats habituate to repeated isolation, but only adolescents sensitize to partner unfamiliarity. Horm. Behav. 2015;69:16–30.
Zhu X, Grace AA. Sex- and exposure age-dependent effects of adolescent stress on ventral tegmental area dopamine system and its afferent regulators. Mol. Psychiatry. 2023;28:611–24.
Fuller JL, Clark LD. Genetic and treatment factors modifying the postisolation syndrome in dogs. J. Comp. Physiol. Psychol. 1966;61:251–7.
Andreasen NC, Olsen S. Negative v positive schizophrenia. Definition and validation. Arch. Gen. Psychiatry. 1982;39:789–94.
Kirkpatrick B, Fenton WS, Carpenter WT Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr. Bull. 2006;32:214–9.
Schenkel LS, Silverstein SM. Dimensions of premorbid functioning in schizophrenia: a review of neuromotor, cognitive, social, and behavioral domains. Genet Soc. Gen. Psychol. Monogr. 2004;130:241–70.
Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr. Res. 2009;110:1–23.
Green MF, Horan WP, Lee J, McCleery A, Reddy LF, Wynn JK. Social Disconnection in Schizophrenia and the General Community. Schizophr. Bull. 2018;44:242–9.
Fulford D, Campellone T, Gard DE. Social motivation in schizophrenia: How research on basic reward processes informs and limits our understanding. Clin. Psychol. Rev. 2018;63:12–24.
Green MF, Horan WP, Lee J. Nonsocial and social cognition in schizophrenia: current evidence and future directions. World Psychiatry. 2019;18:146–61.
Catalano LT, Wynn JK, Green MF, Gold JM. Reduced neural activity when anticipating social versus nonsocial rewards in schizophrenia: Preliminary evidence from an ERP study. Schizophr. Res. 2022;246:7–16.
Powell SB, Swerdlow NR. The relevance of animal models of social isolation and social motivation for understanding schizophrenia: review and future directions. Schizophr. Bull. 2023;49:1112–26.
Macdonald EM, Hayes RL, Baglioni AJ Jr. The quantity and quality of the social networks of young people with early psychosis compared with closely matched controls. Schizophr. Res. 2000;46:25–30.
Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr. Bull. 2007;33:1013–22.
Bleuler M, Bleuler R. Dementia praecox oder die Gruppe der Schizophrenien: Eugen Bleuler. Br. J. Psychiatry. 1986;149:661–2.
K Conrad, Die beginnende Schizophrenie (G. Thieme, Stuttgart,, 1958), 165.
E Kraepelin, RM Barclay, GM Robertson, Dementia præcox and paraphrenia (E. & S. Livingstone, Edinburgh, 1919), pp. 1 p. l., x. 331.
E Minkowski, La schizophrénie; psychopathologie des schizoïdes et des schizophrènes, Bibliothèque scientifique (Payot, Paris, 1927), pp. 2 p[Cognitive remediation: a new approach for treating schizophrenia], Revue medicale de la Suisse romande, 2004, 124, -9 l., 268
Bucci P, Galderisi S, Mucci A, Rossi A, Rocca P, Bertolino A, et al. Premorbid academic and social functioning in patients with schizophrenia and its associations with negative symptoms and cognition. Acta Psychiatr. Scand. 2018;138:253–66.
Chau AKC, Zhu C, So SH. Loneliness and the psychosis continuum: a meta-analysis on positive psychotic experiences and a meta-analysis on negative psychotic experiences. Int Rev. Psychiatry. 2019;31:471–90.
Gottesman II, Shields J. A polygenic theory of schizophrenia. Proc. Natl. Acad. Sci. USA. 1967;58:199–205.
Jonas KG, Lencz T, Li K, Malhotra AK, Perlman G, Fochtmann LJ, et al. Schizophrenia polygenic risk score and 20-year course of illness in psychotic disorders. Transl. Psychiatry. 2019;9:300.
Jones HJ, Stergiakouli E, Tansey KE, Hubbard L, Heron J, Cannon M, et al. Phenotypic manifestation of genetic risk for schizophrenia during adolescence in the general population. JAMA Psychiatry. 2016;73:221–8.
Lencz T, Knowles E, Davies G, Guha S, Liewald DC, Starr JM, et al. Molecular genetic evidence for overlap between general cognitive ability and risk for schizophrenia: a report from the Cognitive Genomics consorTium (COGENT). Mol. Psychiatry. 2014;19:168–74.
Zammit S, Hamshere M, Dwyer S, Georgiva L, Timpson N, Moskvina V, et al. A population-based study of genetic variation and psychotic experiences in adolescents. Schizophr. Bull. 2014;40:1254–62.
Abplanalp SJ, Green MF, Wynn JK, Eisenberger NI, Horan WP, Lee J, et al. Using machine learning to understand social isolation and loneliness in schizophrenia, bipolar disorder, and the community. Schizophrenia. 2024;10:88.
Socrates AJ, Mullins N, Gur RC, Gur RE, Stahl E, O'Reilly PF, et al. Polygenic risk of social isolation behavior and its influence on psychopathology and personality. Mol. Psychiatry. 2024;29:3599–606.
Andreu-Bernabeu Á, Díaz-Caneja CM, Costas J, De Hoyos L, Stella C, Gurriarán X, et al. Polygenic contribution to the relationship of loneliness and social isolation with schizophrenia. Nat. Commun. 2022;13:51.
Day FR, Ong KK, Perry JRB. Elucidating the genetic basis of social interaction and isolation. Nat. Commun. 2018;9:2457.
Brown AS. The environment and susceptibility to schizophrenia. Prog. Neurobiol. 2011;93:23–58.
Bhugra D. Migration and mental health. Acta Psychiatr. Scand. 2004;109:243–58.
Malzberg B. Mental disease among foreign-born in Canada, 1950-2, in relation to period of immigration. Am. J. Psychiatry. 1964;120:971–3.
Ø Ødegård, Emigration and insanity (Levin & Munksgaard, Copenhagen, 1932), 206.
Pedersen CB, Mortensen PB. Evidence of a dose-response relationship between urbanicity during upbringing and schizophrenia risk. Arch. Gen. Psychiatry. 2001;58:1039–46.
Ochnik D, Bulawa B, Nagel P, Gachowski M, Budzinski M. Urbanization, loneliness and mental health model - A cross-sectional network analysis with a representative sample. Sci. Rep. 2024;14:24974.
Ku BS, Addington J, Bearden CE, Cadenhead KS, Cannon TD, Compton MT, et al. Associations between childhood area-level social fragmentation, maladaptation to school, and social functioning among healthy youth and those at clinical high risk for psychosis. Schizophr. Bull. 2023;49:1437–46.
McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch. Gen. Psychiatry. 2000;57:637–48.
Selemon LD, Zecevic N. Schizophrenia: a tale of two critical periods for prefrontal cortical development. Transl. Psychiatry. 2015;5:e623.
Rapoport JL, Giedd JN, Blumenthal J, Hamburger S, Jeffries N, Fernandez T, et al. Progressive cortical change during adolescence in childhood-onset schizophrenia. A longitudinal magnetic resonance imaging study. Arch. Gen. Psychiatry. 1999;56:649–54.
Wible CG, Anderson J, Shenton ME, Kricun A, Hirayasu Y, Tanaka S, et al. Prefrontal cortex, negative symptoms, and schizophrenia: an MRI study. Psychiatry Res. 2001;108:65–78.
Zipursky RB, Lim KO, Sullivan EV, Brown BW, Pfefferbaum A. Widespread cerebral gray matter volume deficits in schizophrenia. Arch. Gen. Psychiatry. 1992;49:195–205.
Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc. Natl. Acad. Sci. USA. 2001;98:11650–5.
Duncan LE, Li T, Salem M, Li W, Mortazavi L, Senturk H, et al. Mapping the cellular etiology of schizophrenia and complex brain phenotypes. Nat. Neurosci. 2025;28:248–58.
Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, et al. Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiatry. 2015;72:882–91.
Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr. Psychiatry Rep. 2010;12:335–44.
Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J. Neurosci. 2003;23:6315–26.
Gonzalez-Burgos G, Lewis DA. NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr. Bull. 2012;38:950–7.
Dienel SJ, Dowling KF, Barile Z, Bazmi HH, Liu A, Vespoli JC, et al. Diagnostic specificity and association with cognition of molecular alterations in prefrontal Somatostatin neurons in schizophrenia. JAMA Psychiatry. 2023;80:1235–45.
Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb. Cortex. 2008;18:1575–87.
Singh T, Poterba T, Curtis D, Akil H, Al Eissa M, Barchas JD, et al. Rare coding variants in ten genes confer substantial risk for schizophrenia. Nature. 2022;604:509–16.
Trubetskoy V, Pardiñas AF, Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604:502–8.
Hansen KB, Wollmuth LP, Bowie D, Furukawa H, Menniti FS, Sobolevsky AI, et al. Structure, function, and pharmacology of glutamate receptor ion channels. Pharm. Rev. 2021;73:298–487.
Farsi Z, Nicolella A, Simmons SK, Aryal S, Shepard N, Brenner K, et al. Brain-region-specific changes in neurons and glia and dysregulation of dopamine signaling in Grin2a mutant mice. Neuron. 2023;111:3378–96 e3379.
Mielnik CA, Binko MA, Chen Y, Funk AJ, Johansson EM, Intson K, et al. Consequences of NMDA receptor deficiency can be rescued in the adult brain. Mol. Psychiatry. 2021;26:2929–42.
Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat. Neurosci. 2010;13:76–83.
Jiang Z, Rompala GR, Zhang S, Cowell RM, Nakazawa K. Social isolation exacerbates schizophrenia-like phenotypes via oxidative stress in cortical interneurons. Biol. Psychiatry. 2013;73:1024–34.
Oshima I, Mino Y, Inomata Y. Effects of environmental deprivation on negative symptoms of schizophrenia: a nationwide survey in Japan’s psychiatric hospitals. Psychiatry Res. 2005;136:163–71.
Hoffman RE. A social deafferentation hypothesis for induction of active schizophrenia. Schizophr. Bull. 2007;33:1066–70.
Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, et al. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375:482–4.
McCrory E, Foulkes L, Viding E. Social thinning and stress generation after childhood maltreatment: a neurocognitive social transactional model of psychiatric vulnerability. Lancet Psychiatry. 2022;9:828–37.
Miller P, Lawrie SM, Hodges A, Clafferty R, Cosway R, Johnstone EC. Genetic liability, illicit drug use, life stress and psychotic symptoms: preliminary findings from the Edinburgh study of people at high risk for schizophrenia. Soc. Psychiatry Psychiatr. Epidemiol. 2001;36:338–42.
Serban G. Relationship of mental status, functioning and stress to readmission of schizophrenics. Br. J. Soc. Clin. Psychol. 1975;14:291–301.
Chan S, Liu T, Wong A, Wong G, Hsiao J, Hui C, et al. Self-referential gaze perception of patients with schizophrenia and its relationship with symptomatology and cognitive functions. Schizophr. Res. 2021;228:288–94.
Mancuso F, Horan WP, Kern RS, Green MF. Social cognition in psychosis: multidimensional structure, clinical correlates, and relationship with functional outcome. Schizophr. Res. 2011;125:143–51.
Devoe DJ, Peterson A, Addington J. Negative symptom interventions in youth at risk of psychosis: a systematic review and network meta-analysis. Schizophr. Bull. 2018;44:807–23.
Kantrowitz JT, Woods SW, Petkova E, Cornblatt B, Corcoran CM, Chen H, et al. D-serine for the treatment of negative symptoms in individuals at clinical high risk of schizophrenia: a pilot, double-blind, placebo-controlled, randomised parallel group mechanistic proof-of-concept trial. Lancet Psychiatry. 2015;2:403–12.
Tseng PT, Zeng BS, Hung CM, Liang CS, Stubbs B, Carvalho AF, et al. Assessment of noninvasive brain stimulation interventions for negative symptoms of schizophrenia: a systematic review and network meta-analysis. JAMA Psychiatry. 2022;79:770–9.
Hadar R, Bikovski L, Soto-Montenegro ML, Schimke J, Maier P, Ewing S, et al. Early neuromodulation prevents the development of brain and behavioral abnormalities in a rodent model of schizophrenia. Mol. Psychiatry. 2018;23:943–51.
McGlashan TH, Zipursky RB, Perkins D, Addington J, Miller T, Woods SW, et al. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am. J. Psychiatry. 2006;163:790–9.
McGorry PD, Yung AR, Phillips LJ, Yuen HP, Francey S, Cosgrave EM, et al. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch. Gen. Psychiatry. 2002;59:921–8.
Cornblatt BA, Lencz T, Smith CW, Olsen R, Auther AM, Nakayama E, et al. Can antidepressants be used to treat the schizophrenia prodrome? Results of a prospective, naturalistic treatment study of adolescents. J. Clin. Psychiatry. 2007;68:546–57.
Schmidt SJ, Schultze-Lutter F, Schimmelmann BG, Maric NP, Salokangas RK, Riecher-Rössler A, et al. EPA guidance on the early intervention in clinical high risk states of psychoses. Eur. Psychiatry. 2015;30:388–404.
Antal A, Fischer T, Saiote C, Miller R, Chaieb L, Wang DJ, et al. Transcranial electrical stimulation modifies the neuronal response to psychosocial stress exposure. Hum. Brain Mapp. 2014;35:3750–9.
Craig TK, Rus-Calafell M, Ward T, Leff JP, Huckvale M, Howarth E, et al. AVATAR therapy for auditory verbal hallucinations in people with psychosis: a single-blind, randomised controlled trial. Lancet Psychiatry. 2018;5:31–40.
Percie du Sert O, Potvin S, Lipp O, Dellazizzo L, Laurelli M, Breton R, et al. Virtual reality therapy for refractory auditory verbal hallucinations in schizophrenia: A pilot clinical trial. Schizophr. Res. 2018;197:176–81.
Okuyama T. Social memory engram in the hippocampus. Neurosci. Res. 2018;129:17–23.
Smith AS, Williams Avram SK, Cymerblit-Sabba A, Song J, Young WS. Targeted activation of the hippocampal CA2 area strongly enhances social memory. Mol. Psychiatry. 2016;21:1137–44.
Sun Q, Li X, Li A, Zhang J, Ding Z, Gong H, Luo Q. Ventral hippocampal-prefrontal interaction affects social behavior via parvalbumin positive neurons in the medial prefrontal cortex. iScience. 2020;23:100894.
Choi TY, Jeon H, Jeong S, Kim EJ, Kim J, Jeong YH, et al. Distinct prefrontal projection activity and transcriptional state conversely orchestrate social competition and hierarchy. Neuron. 2024;112:611–27 e618.
N Rigney, GJ de Vries, A Petrulis, LJ Young, Oxytocin, Vasopressin, and social behavior: from neural circuits to clinical opportunities. Endocrinology. 2022;163:bqac111.
Tan Y, Singhal SM, Harden SW, Cahill KM, Nguyen DM, Colon-Perez LM, et al. Oxytocin receptors are expressed by glutamatergic prefrontal cortical neurons that selectively modulate social recognition. J. Neurosci. 2019;39:3249–63.
Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–6.
DJ Christoffel, JJ Walsh, P Hoerbelt, BD Heifets, P Llorach, RC Lopez, et al. Selective filtering of excitatory inputs to nucleus accumbens by dopamine and serotonin. Proc. Natl Acad. Sci. USA. 2021:118:e2106648118.
Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, Tye KM. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience. 2016;321:197–209.
Kim H, Lim CS, Kaang BK. Neuronal mechanisms and circuits underlying repetitive behaviors in mouse models of autism spectrum disorder. Behav. Brain Funct. 2016;12:3.
Cao P, Liu Y, Ni Z, Zhang M, Wei HR, Liu A, et al. Rescue-like behavior in a bystander mouse toward anesthetized conspecifics promotes arousal via a tongue-brain connection. Sci. Adv. 2025;11:eadq3874.
Dai B, Sun F, Tong X, Ding Y, Kuang A, Osakada T, et al. Responses and functions of dopamine in nucleus accumbens core during social behaviors. Cell Rep. 2022;40:111246.
Terranova JI, Yokose J, Osanai H, Marks WD, Yamamoto J, Ogawa SK, Kitamura T. Hippocampal-amygdala memory circuits govern experience-dependent observational fear. Neuron. 2022;110:1416–31.
Funding
This work was supported by the National Institute of Mental Health: R01MH118297 to Hirofumi Morishita and F31MH127805 to Michael B. Leventhal, National Institute on Drug Abuse: R34DA061263 to Hirofumi Morishita, the Simons Foundation/SFARI (grant no. 610850) to Hirofumi Morishita, and the Heiwa Nakajima Foundation to Ayako Kawatake-Kuno.
Author information
Authors and Affiliations
Contributions
Ayako Kawatake-Kuno, Michael B. Leventhal, and Hirofumi Morishita conceptualized the research goals and aims of this manuscript. Ayako Kawatake-Kuno and Michael B. Leventhal curated and analyzed references used to inform this manuscript, with help from Hirofumi Morishita acquired funding to write this manuscript. Hirofumi Morishita supervised this project. Visualizations were created by Ayako Kawatake-Kuno and Michael B. Leventhal revised with feedback from Hirofumi Morishita. The original draft of this manuscript was produced by Ayako Kawatake-Kuno and Michael B. Leventhal. The manuscript was reviewed and edited by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kawatake-Kuno, A., Leventhal, M.B. & Morishita, H. Impact of early social isolation on social circuits and behavior: relevance to schizophrenia. Neuropsychopharmacol. 51, 46–56 (2026). https://doi.org/10.1038/s41386-025-02156-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-025-02156-6
This article is cited by
-
Social environment as a determinant of mismatch negativity in early psychosis
Neuropsychopharmacology (2026)
-
Trajectories of mental health and mental illness: where we are now and where we go next
Neuropsychopharmacology (2026)