Abstract
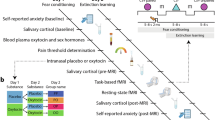
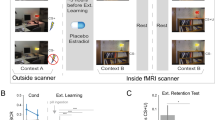
Estradiol (E2), whether endogenous or administered via oral contraceptives (OCs), modulates fear regulation. However, its role in contextual fear signaling, critical for distinguishing threat from safety, remains poorly understood in humans. While prior human studies focused on cue-related extinction recall, animal research suggests that a single high-dose of exogenous E2 generalizes fear to safe contexts. This study tested whether hormonal status and OC use influence contextual fear responses in humans, and whether OC-related effects are long-lasting and dose-dependent. In a two-day fear conditioning and extinction protocol, 147 healthy participants (men, cycling women, OC users) underwent fear conditioning in a threat-associated context (CX + ) and extinction in a safety-associated context (CX-). On day 2, cues were presented in both contexts. Fear return to each context was assessed via skin conductance responses and brain activations. Groups were formed sequentially using the same participants: (A) by endogenous/exogenous E2 status, (B) by OC use history, and (C) by current ethinyl estradiol (EE) dose. CX- fear returns were elevated in current (p = .015, d = 0.64) and past OC users (p = .014, d = 0.83) compared to never users. High-EE, but not low-EE users, showed greater fear returns than never users (p = .021, d = 0.81). Biological sex and endogenous E2 were unrelated to contextual fear. Across participants, CX- fear returns were negatively associated with hippocampal and ventromedial prefrontal cortex activation. OC use, particularly at higher EE doses, may impair retrieval of safety signals from context, with effects persisting beyond discontinuation. These findings highlight exogenous hormones as a modulator of contextual fear regulation in women.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The raw data supporting the conclusions of this article will be made available by the authors upon request.
References
Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–28.
Rougemont-Bücking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, et al. Altered processing of contextual information during fear extinction in PTSD: An fMRI study. CNS Neurosci Ther. 2011;17:227–36.
Keiser AA, Turnbull LM, Darian MA, Feldman DE, Song I, Tronson NC. Sex differences in context fear generalization and recruitment of hippocampus and amygdala during retrieval. Neuropsychopharmacology. 2017;42:397–407.
Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–82.
Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–54.
Bian X-L, Qin C, Cai C-Y, Zhou Y, Tao Y, Lin Y-H, et al. Anterior cingulate cortex to ventral hippocampus circuit mediates contextual fear generalization. J Neurosci. 2019;39:5728–39.
Day HLL, Stevenson CW. The neurobiological basis of sex differences in learned fear and its inhibition. Eur J Neurosci. 2020;52:2466–86.
Glenn DE, Risbrough VB, Simmons AN, Acheson DT, Stout DM The future of contextual fear learning for PTSD research: a methodological review of neuroimaging studies. Behav Neurobiol PTSD. 2017;207-28.
Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci. 2006;26:9503–11.
Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–7.
Garfinkel SN, Abelson JL, King AP, Sripada RK, Wang X, Gaines LM, et al. Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal. J Neurosci. 2014;34:13435–43.
Jovanovic T, Kazama A, Bachevalier J, Davis M. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology. 2012;62:695–704.
Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, et al. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety. 2010;27:244–51.
Jovanovic T, Norrholm SD, Blanding NQ, Phifer JE, Weiss T, Davis M, et al. Fear potentiation is associated with hypothalamic–pituitary–adrenal axis function in PTSD. Psychoneuroendocrinology. 2010;35:846–57.
Liberzon I, Abelson JL. Context processing and the neurobiology of post-traumatic stress disorder. Neuron. 2016;92:14–30.
Altemus M, Sarvaiya N, Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol. 2014;35:320–30.
Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen H. Twelve‐month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169–84.
McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res. 2011;45:1027–35.
Cover KK, Maeng LY, Lebron-Milad K, Milad MR. Mechanisms of estradiol in fear circuitry: implications for sex differences in psychopathology. Transl Psychiatry. 2014;4:e422.
Hammoud MZ, Foa EB, Milad MR. Oestradiol, threat conditioning and extinction, post-traumatic stress disorder, and prolonged exposure therapy: A common link. J Neuroendocrinol. 2020;32:e12800.
Lebron-Milad K, Graham BM, Milad MR. Low estradiol levels: A vulnerability factor for the development of posttraumatic stress disorder. Biol Psychiatry. 2012;72:6–7.
Asok A, Hijazi J, Harvey LR, Kosmidis S, Kandel ER, Rayman JB. Sex differences in remote contextual fear generalization in mice. Front Behav Neurosci. 2019;13:56.
Lynch JF, Cullen PK, Jasnow AM, Riccio DC. Sex differences in the generalization of fear as a function of retention intervals. Learn Mem. 2013;20:628–32.
Adkins JM, Lynch JF, Hagerdorn P, Esterhuizen M, Jasnow AM. Anterior cingulate cortex and dorsal hippocampal glutamate receptors mediate generalized fear in female rats. Psychoneuroendocrinology. 2019;107:109–18.
Lynch JF, Winiecki P, Vanderhoof T, Riccio DC, Jasnow AM. Hippocampal cytosolic estrogen receptors regulate fear generalization in females. Neurobiol Learn Mem. 2016;130:83–92.
Lynch JF, Dejanovic D, Winiecki P, Mulvany J, Ortiz S, Riccio DC, et al. Activation of ERβ modulates fear generalization through an effect on memory retrieval. Horm Behav. 2014;66:421–9.
Dickey RP, Seymour ML Managing contraceptive pill patients and other hormonal contraceptives. Revised seventeenth edition. Fort Collins, CO: EMIS Inc. Medical Publishers; 2021.
Dragoman MV. The combined oral contraceptive pill- recent developments, risks and benefits. Best Pract Res Clin Obstet Gynaecol. 2014;28:825–34.
United Nations. Contraceptive use by method 2019: Data booklet. 2019.
Rivera R, Yacobson I, Grimes D. The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. Am J Obstet Gynecol. 1999;181:1263–9.
Zimmerman Y, Eijkemans MJC, Coelingh Bennink HJT, Blankenstein MA, Fauser BCJM. The effect of combined oral contraception on testosterone levels in healthy women: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:76–105.
Reed BG, Carr BR The normal menstrual cycle and the control of ovulation. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, et al., editors. Endotext, South Dartmouth (MA): MDText.com, Inc.; 2000.
Milad MR, Orr SP, Pitman RK, Rauch SL. Context modulation of memory for fear extinction in humans. Psychophysiology. 2005;42:456–64.
Bouton ME. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–86.
Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: Behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–60.
Harris JA, Jones ML, Bailey GK, Westbrook RF. Contextual control over conditioned responding in an extinction paradigm. J Exp Psychol Anim Behav Process. 2000;26:174–85.
Lonsdorf TB, Menz MM, Andreatta M, Fullana MA, Golkar A, Haaker J, et al. Don’t fear ‘fear conditioning’: Methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neurosci Biobehav Rev. 2017;77:247–85.
Bierwirth P, Sperl MFJ, Antov MI, Stockhorst U Prefrontal theta oscillations are modulated by estradiol-status during fear recall and extinction recall. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021:S2451902221000574.
Davignon LM, Brouillard A, Devine S, Taschereau-Dumouchel V, Roy M, Marin MF. (under review). Associations between past and current use of oral contraceptives and fear regulation.
Graham BM, Milad MR. Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biol Psychiatry. 2013;73:371–8.
Li SH, Graham BM. Estradiol is associated with altered cognitive and physiological responses during fear conditioning and extinction in healthy and spider phobic women. Behav Neurosci. 2016;130:614–23.
Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, et al. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–8.
White EC, Graham BM. Estradiol levels in women predict skin conductance response but not valence and expectancy ratings in conditioned fear extinction. Neurobiol Learn Mem. 2016;134:339–48.
Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, et al. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiatry. 2011;70:920–7.
Milad MR, Goldstein JM, Orr SP, Wedig MM, Klibanski A, Pitman RK, et al. Fear conditioning and extinction: Influence of sex and menstrual cycle in healthy humans. Behav Neurosci. 2006;120:1196–203.
Chang Y-J, Yang C, Liang Y, Yeh C, Huang C, Hsu K. Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor β. Hippocampus. 2009;19:1142–50.
Graham BM, Milad MR. Inhibition of estradiol synthesis impairs fear extinction in male rats. Learn Mem. 2014;21:347–50.
Maeng LY, Cover KK, Taha MB, Landau AJ, Milad MR, Lebrón-Milad K. Estradiol shifts interactions between the infralimbic cortex and central amygdala to enhance fear extinction memory in female rats. J Neurosci Res. 2017;95:163–75.
Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164:887–95.
Rey CD, Lipps J, Shansky RM. Dopamine D1 receptor activation rescues extinction impairments in low-estrogen female rats and induces cortical layer-specific activation changes in prefrontal–amygdala circuits. Neuropsychopharmacology. 2014;39:1282–9.
Wen Z, Hammoud MZ, Scott JC, Jimmy J, Brown L, Marin M-F, et al. Impact of exogenous estradiol on task-based and resting-state neural signature during and after fear extinction in healthy women. Neuropsychopharmacology. 2021;46:2278–87.
Wegerer M, Kerschbaum H, Blechert J, Wilhelm FH. Low levels of estradiol are associated with elevated conditioned responding during fear extinction and with intrusive memories in daily life. Neurobiol Learn Mem. 2014;116:145–54.
Lonsdorf TB, Haaker J, Schümann D, Sommer T, Bayer J, Brassen S, et al. Sex differences in conditioned stimulus discrimination during context-dependent fear learning and its retrieval in humans: The role of biological sex, contraceptives and menstrual cycle phases. J Psychiatry Neurosci. 2015;40:368–75.
Graham BM. The impact of hormonal contraceptives on anxiety treatments: From preclinical models to clinical settings. Front Neuroendocrinol. 2022;67:101030.
Marschner A, Kalisch R, Vervliet B, Vansteenwegen D, Büchel C. Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. J Neurosci. 2008;28:9030–6.
Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–85.
Lacasse JM, Gomez-Perales E, Brake WG. Modeling hormonal contraception in female rats: A framework for studies in behavioral neurobiology. Front Neuroendocrinol. 2022;67:101020.
Casto KV, Jordan T, Petersen N. Hormone-based models for comparing menstrual cycle and hormonal contraceptive effects on human resting-state functional connectivity. Front Neuroendocrinol. 2022;67:101036.
Hampson E. Oral contraceptives in the central nervous system: Basic pharmacology, methodological considerations, and current state of the field. Front Neuroendocrinol. 2023;68:101040.
Anderl C, Li G, Chen FS. Oral contraceptive use in adolescence predicts lasting vulnerability to depression in adulthood. J Child Psychol Psychiatry. 2020;61:148–56.
Brouillard A, Davignon LM, Fortin J, Marin MF. A year through the COVID-19 pandemic: Deleterious impact of hormonal contraception on psychological distress in women. Front Psychiatry. 2022;13:835857.
Egan KR, Gleason CE. Longer duration of hormonal contraceptive use predicts better cognitive outcomes later in life. J Womens Health Larchmt. 2012;21:1259–66.
Karim R, Dang H, Henderson VW, Hodis HN, St John J, Brinton RD, et al. Effect of reproductive history and exogenous hormone use on cognitive function in mid- and late life. J Am Geriatr Soc. 2016;64:2448–56.
Pletzer B, Harris T, Hidalgo-Lopez E Previous contraceptive treatment relates to grey matter volumes in the hippocampus and basal ganglia. Sci Rep. 2019;9.
Brouillard A, Davignon L-M, Turcotte A-M, Marin M-F. Morphologic alterations of the fear circuitry: The role of sex hormones and oral contraceptives. Front Endocrinol. 2023;14:1228504.
Brouillard A, Davignon L, Vachon‐Presseau É, Roy M, Marin M. Starting the pill during adolescence: Age of onset and duration of use influence morphology of the hippocampus and ventromedial prefrontal cortex. Eur J Neurosci. 2024;60:5876–99.
Brouillard A, Davignon L-M, Cernik R, Giguère C-É, Findlay H, Juster R-P, et al. Comparing immunoassay and mass spectrometry techniques for salivary sex hormone analysis. Psychoneuroendocrinology. 2025;174:107379.
Davignon L-M, Brouillard A, Juster R-P, Marin M-F. The role of sex hormones, oral contraceptive use, and its parameters on visuospatial abilities, verbal fluency, and verbal memory. Horm Behav. 2024;157:105454.
Linnman C, Zeidan MA, Pitman RK, Milad MR. Resting cerebral metabolism correlates with skin conductance and functional brain activation during fear conditioning. Biol Psychol. 2012;89:450–9.
Marin M-F, Song H, VanElzakker MB, Staples-Bradley LK, Linnman C, Pace-Schott EF, et al. Association of resting Metabolism in the fear neural network with extinction recall activations and clinical measures in trauma-exposed individuals. Am J Psychiatry. 2016;173:930–8.
Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007;62:1191–4.
Benedek M, Kaernbach C. A continuous measure of phasic electrodermal activity. J Neurosci Methods. 2010;190:80–91.
Boucsein W, Fowles D, Grimnes S, Ben-Shakhar G, Roth W, Dawson M, et al. Publication recommendations for electrodermal measurements: Publication standards for EDA. Psychophysiology. 2012;49:1017–34.
Fraser KM, Holland PC. Occasion setting. Behav Neurosci. 2019;133:145–75.
Marin M-F, Barbey F, Rosenbaum BL, Hammoud MZ, Orr SP, Milad MR Absence of conditioned responding in humans: A bad measure or individual differences? Psychophysiology. 2020;57.
Duits P, Cath DC, Lissek S, Hox JJ, Hamm AO, Engelhard IM, et al. Updated meta-analysis of classical fear conditioning in the anxiety disorders. Depress Anxiety. 2015;32:239–53.
Dibbets P, van den Broek A, Evers EAT. Fear conditioning and extinction in anxiety- and depression-prone persons. Memory. 2015;23:350–64.
Goldscheider C, Mosher WD. Patterns of contraceptive use in the United States: The importance of religious factors. Stud Fam Plann. 1991;22:102.
Vishkin A. Variation and consistency in the links between religion and emotion regulation. Curr Opin Psychol. 2021;40:6–9.
Amstadter A. Emotion regulation and anxiety disorders. J Anxiety Disord. 2008;22:211–21.
Hartley CA, Phelps EA. Changing fear: The neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35:136–46.
Watson D, Clark LA, Simms LJ, Kotov R. Classification and assessment of fear and anxiety in personality and psychopathology. Neurosci Biobehav Rev. 2022;142:104878.
R Core Team. R: A language and environment for statistical computing. Vienna, Austria; 2023.
Blaine BE Winsorizing. SAGE Encycl. Educ. Res. Meas. Eval., 2455 Teller Road, Thousand Oaks, California 91320: SAGE Publications, Inc.; 2018.
Bates D, Mächler M, Bolker B, Walker S Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67.
Kuznetsova A, Brockhoff PB, Christensen RHB lmerTest package: Tests in linear mixed effects models. J Stat Softw. 2017;82.
Dawson ME, Schell AM, Filion DL, Berntson GG The Electrodermal System. In: Cacioppo JT, Tassinary LG, Berntson G, editors. Handb. Psychophysiol. 3rd ed. Cambridge: Cambridge University Press; 2007. 157-81.
Lonsdorf TB, Merz CJ, Fullana MA. Fear extinction retention: Is it what we think it is? Biol Psychiatry. 2019;85:1074–82.
Doornweerd AM, Gerritsen L, Montoya ER, Engelhard IM, Baas JMP. Contraceptives and conditioning: Different profiles of fear and expectancy ratings during fear conditioning and extinction according to menstrual cycle phase and hormonal contraceptive use. Biol Psychol. 2025;194:108964.
Lebron-Milad K, Abbs B, Milad MR, Linnman C, Rougemount-Bücking A, Zeidan MA, et al. Sex differences in the neurobiology of fear conditioning and extinction: a preliminary fMRI study of shared sex differences with stress-arousal circuitry. Biol Mood Anxiety Disord. 2012;2:7.
Wen Z, Raio CM, Pace-Schott EF, Lazar SW, LeDoux JE, Phelps EA, et al. Temporally and anatomically specific contributions of the human amygdala to threat and safety learning. Proc Natl Acad Sci. 2022;119:e2204066119.
Cohen J Statistical Power Analysis for the Behavioral Sciences. Routledge; 2013.
Roiser JP, Linden DE, Gorno-Tempini ML, Moran RJ, Dickerson BC, Grafton ST. Minimum statistical standards for submissions to Neuroimage: Clinical. NeuroImage Clin. 2016;12:1045–7.
Barth C, Steele CJ, Mueller K, Rekkas VP, Arélin K, Pampel A, et al. In-vivo dynamics of the human hippocampus across the menstrual cycle. Sci Rep. 2016;6:32833.
Hwang MJ, Zsido RG, Song H, Pace-Schott EF, Miller KK, Lebron-Milad K, et al. Contribution of estradiol levels and hormonal contraceptives to sex differences within the fear network during fear conditioning and extinction. BMC Psychiatry. 2015;15:295.
Mahmoud R, Wainwright SR, Galea LAM. Sex hormones and adult hippocampal neurogenesis: Regulation, implications, and potential mechanisms. Front Neuroendocrinol. 2016;41:129–52.
Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, et al. Oral contraceptive usage alters the effects of cortisol on implicit fear learning. Horm Behav. 2012;62:531–8.
Noachtar IA, Hidalgo-Lopez E, Pletzer B. Duration of oral contraceptive use relates to cognitive performance and brain activation in current and past users. Front Endocrinol. 2022;13:885617.
Protopopescu X, Butler T, Pan H, Root J, Altemus M, Polanecsky M, et al. Hippocampal structural changes across the menstrual cycle. Hippocampus. 2008;18:985–8.
Hoekzema E, Barba-Muller E, Pozzobon C, Picado M, Lucco F, Garcia-Garcia D, et al. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 2017;20:287–96.
Skovlund CW, Mørch LS, Kessing LV, Lidegaard Ø. Association of hormonal contraception with depression. JAMA Psychiatry. 2016;73:1154.
Jeyakumar M, Carlson KE, Gunther JR, Katzenellenbogen JA. Exploration of dimensions of estrogen potency. J Biol Chem. 2011;286:12971–82.
Stanczyk FZ, Archer DF, Bhavnani BR. Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: Pharmacokinetics, pharmacodynamics and risk assessment. Contraception. 2013;87:706–27.
Dickson RB, Eisenfeld AJ. 17 Alpha-ethinyl estradiol is more potent than estradiol in receptor interactions with isolated hepatic parenchymal cells. Endocrinology. 1981;108:1511–8.
Cha J, Greenberg T, Carlson JM, DeDora DJ, Hajcak G, Mujica-Parodi LR. Circuit-wide structural and functional measures predict ventromedial prefrontal cortex fear generalization: Implications for generalized anxiety disorder. J Neurosci. 2014;34:4043–53.
Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci. 2005;102:10706–11.
Winkelmann T, Grimm O, Pohlack ST, Nees F, Cacciaglia R, Dinu-Biringer R, et al. Brain morphology correlates of interindividual differences in conditioned fear acquisition and extinction learning. Brain Struct Funct. 2016;221:1927–37.
Ehlers MR, Nold J, Kuhn M, Klingelhöfer-Jens M, Lonsdorf TB. Revisiting potential associations between brain morphology, fear acquisition and extinction through new data and a literature review. Sci Rep. 2020;10:19894.
Kharabian Masouleh S, Eickhoff SB, Hoffstaedter F, Genon S. Alzheimer’s Disease Neuroimaging Initiative. Empirical examination of the replicability of associations between brain structure and psychological variables. eLife. 2019;8:e43464.
Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93.
Greco JA, Liberzon I. Neuroimaging of fear-associated learning. Neuropsychopharmacology. 2016;41:320–34.
Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–51.
Pletzer B, Winkler-Crepaz K, Hillerer K Progesterone and contraceptive progestin actions on the brain: A systematic review of animal studies and comparison to human neuroimaging studies. Front Neuroendocrinol. 2023:101060.
Griksiene R, Monciunskaite R, Ruksenas O. What is there to know about the effects of progestins on the human brain and cognition? Front Neuroendocrinol. 2022;67:101032.
Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, et al. Neuronal correlates of extinction learning are modulated by sex hormones. Soc Cogn Affect Neurosci. 2012;7:819–30.
Brun CC, Leporé N, Luders E, Chou Y-Y, Madsen SK, Toga AW, et al. Sex differences in brain structure in auditory and cingulate regions. NeuroReport. 2009;20:930–5.
DeCasien AR, Guma E, Liu S, Raznahan A. Sex differences in the human brain: a roadmap for more careful analysis and interpretation of a biological reality. Biol Sex Differ. 2022;13:43.
Liu S, Seidlitz J, Blumenthal JD, Clasen LS, Raznahan A. Integrative structural, functional, and transcriptomic analyses of sex-biased brain organization in humans. Proc Natl Acad Sci. 2020;117:18788–98.
Ruigrok ANV, Salimi-Khorshidi G, Lai M-C, Baron-Cohen S, Lombardo MV, Tait RJ, et al. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 2014;39:34–50.
Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord. 2012;2:9.
Shin LM, Bush G, Milad MR, Lasko NB, Brohawn KH, Hughes KC, et al. Exaggerated activation of dorsal anterior cingulate cortex during cognitive interference: A monozygotic twin study of posttraumatic stress disorder. Am J Psychiatry. 2011;168:979–85.
Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–91.
Andreatta M, Pauli P. Contextual modulation of conditioned responses in humans: A review on virtual reality studies. Clin Psychol Rev. 2021;90:102095.
Zettermark S, Perez Vicente R, Merlo J. Hormonal contraception increases the risk of psychotropic drug use in adolescent girls but not in adults: A pharmacoepidemiological study on 800 000 Swedish women. PLOS ONE. 2018;13:e0194773.
Chen FS, Zareian B, Nelson MA, Edwards N, Anderl C Research Review: Are sampling biases masking long‐term effects of hormonal contraceptive use in adolescence on risk for depression? J Child Psychol Psychiatry. 2025:jcpp.14180.
Charlton BM, Janiak E, Gaskins AJ, DiVasta AD, Jones RK, Missmer SA, et al. Contraceptive use by women across different sexual orientation groups. Contraception. 2019;100:202–8.
Acknowledgements
We are grateful to Anne-Marie Turcotte, Alexandra Laliberté, Shany Girouard, Annabelle Guérard, and Gabrielle Wester for their contributions to data collection. We also extend our thanks to Simon Lafrance, Jeni Chen, Charles-Édouard Giguère, and Eugénie Samson-Daoust for their support with statistical analyses.
Funding
This research was funded by the Canadian Institutes of Health Research (CIHR, grant #169080), pilot funding from the Quebec Bio-Imaging Network (grant #35450), and support from the Research Center of the Institut universitaire en santé mentale de Montréal. MFM received a salary award from the Fonds de recherche du Québec – Santé (FRQS) between 2018 and 2022 and currently holds a Canada Research Chair. AB and LMD are recipients of CIHR doctoral scholarships and previously held master’s-level awards from FRQS and CIHR.
Author information
Authors and Affiliations
Contributions
AB and MFM contributed to the conception and design of the study. LMD and AB managed data collection. LMD organized the database, performed the statistical analyses, and wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Davignon, LM., Brouillard, A. & Marin, MF. Lasting effects of oral contraceptives on fear responses to a safe context. Neuropsychopharmacol. (2025). https://doi.org/10.1038/s41386-025-02208-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41386-025-02208-x