Abstract
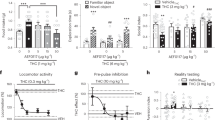
Cannabis products premixed with caffeine are increasingly present in the United States marketplace. Despite emergence of this product class, no human laboratory data have directly evaluated the isolated impact of caffeine on Δ9-tetrahydrocannabinol (Δ9-THC) effects as well as additional impacts of other common co-administered cannabinoids. This double-blind, randomized, placebo-controlled, within-subject crossover study evaluated potential pharmacodynamic and pharmacokinetic interactions between/among Δ9-THC, caffeine, and cannabidiol (CBD). Participants (N = 20; 10 men/10 women) completed outpatient laboratory sessions in which oral Δ9-THC (7.5 mg cumulative), caffeine (180 mg cumulative), and/or CBD (105 mg cumulative) were co-administered in a cumulative dosing design. Primary outcomes included subjective effects indicative of abuse liability (e.g., drug high), performance effects that underlie safety risk (e.g., simulated driving), and plasma cannabinoid/caffeine concentrations. Caffeine co-administration produced minimal changes in Δ9-THC-induced subjective effects, performance, or metabolism, although signals for perceived driving impairment were observed. In contrast, CBD, when co-administered with Δ9-THC and caffeine increased outcomes associated with abuse liability (e.g., drug high, p = 0.002) and performance impairment versus Δ9-THC alone. CBD also increased plasma Δ9-THC (p = 0.004) and 11-OH-Δ9-THC (p < 0.001) concentrations compared with dose conditions without CBD co-administration. These data provide the first direct assessment of the pharmacodynamic and pharmacokinetic effects of Δ9-THC and caffeine when co-administered in humans. The robust alteration of Δ9-THC-induced effects and Δ9-THC pharmacokinetics by CBD further emphasizes the importance of considering full cannabinoid profiles. Broadly, these data highlight the importance of considering drug combinations and interactions in future cannabis regulatory decision-making.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Data and source code for analysis are available upon request.
References
Business Insider. I tried one of the first caffeinated cannabis drinks, and it gave me a giddy buzz. 2021. https://www.businessinsider.com/i-drank-caffeinated-cannabis-seltzer-was-perfect-for-going-out-2021-8.
Panlilio LV, Ferré S, Yasar S, Thorndike EB, Schindler CW, Goldberg SR. Combined effects of THC and caffeine on working memory in rats. Br J Pharm. 2012;165:2529–38.
Justinová Z, Ferré S, Redhi GH, Mascia P, Stroik J, Quarta D, et al. Reinforcing and neurochemical effects of cannabinoid CB1 receptor agonists, but not cocaine, are altered by an adenosine A2A receptor antagonist. Addiction Biol. 2011;16:405–15.
Ferré S, Lluís C, Justinova Z, Quiroz C, Orru M, Navarro G, et al. Adenosine–cannabinoid receptor interactions. Implications for striatal function. Br J Pharm. 2010;160:443–53.
Ferreira S, Gonçalves F, Marques J, Tomé ÂR, Rodrigues R, Nunes-Correia I, et al. Presynaptic adenosine A2A receptors dampen cannabinoid CB 1 receptor-mediated inhibition of corticostriatal glutamatergic transmission. Br J Pharm. 2015;172:1074–86.
Tebano MT, Martire A, Popoli P. Adenosine A2A–cannabinoid CB1 receptor interaction: an integrative mechanism in striatal glutamatergic neurotransmission. Brain Res. 2012;1476:108–18.
Moreno E, Chiarlone A, Medrano M, Puigdellívol M, Bibic L, Howell LA, et al. Singular location and signaling profile of adenosine A2A-cannabinoid CB1 receptor heteromers in the dorsal striatum. Neuropsychopharmacology. 2018;43:964–77.
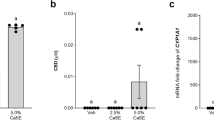
Thai C, Tayo B, Critchley D. A phase 1 open-label, fixed-sequence pharmacokinetic drug interaction trial to investigate the effect of cannabidiol on the CYP1A2 Probe caffeine in healthy subjects. Clin Pharm Drug Dev. 2021;10:1279–89.
Ferretti ML, Gustin ND, Sokol CM, Zamarripa CA, Feldner MT, Bonn-Miller MO, et al. A preliminary investigation of the simultaneous effects of cannabidiol and caffeine. Exp Clin Psychopharmacol. 2024;32:475.
Bansal S, Zamarripa CA, Spindle TR, Weerts EM, Thummel KE, Vandrey R, et al. Evaluation of cytochrome P450-mediated cannabinoid-drug interactions in healthy adult participants. Clin Pharm Therapeutics. 2023;114:693–703.
Zamarripa CA, Spindle TR, Surujunarain R, Weerts EM, Bansal S, Unadkat JD, et al. Assessment of orally administered Δ9-tetrahydrocannabinol when coadministered with cannabidiol on Δ9-tetrahydrocannabinol pharmacokinetics and pharmacodynamics in healthy adults: a randomized clinical trial. JAMA Netw Open. 2023;6:e2254752–e52.
Christians U, Schmitz V, Haschke M. Functional interactions between P-glycoprotein and CYP3A in drug metabolism. Expert Opin drug Metab Toxicol. 2005;1:641–54.
Zhu H-J, Wang J-S, Markowitz JS, Donovan JL, Gibson BB, Gefroh HA, et al. Characterization of P-glycoprotein inhibition by major cannabinoids from marijuana. J Pharm Exp Therapeutics. 2006;317:850–57.
Food and Drug Administration. Assessment of Abuse Potential of Drugs. (Food and Drug Administration, 2017).
Spielberger, C. D., Gorsuch, R. L. & Lushene, R. E. The State-Trait Anxiety Inventory (Test Manual). Palo Alto, California: Consulting Psychologists Press, 1970.
Reynolds CR. Forward and backward memory span should not be combined for clinical analysis. Arch Clin Neuropsychol. 1997;12:29–40.
McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test. Behav Res Methods Instrum. 1982;14:463–66.
Spindle TR, Martin EL, Grabenauer M, Woodward T, Milburn MA, Vandrey R. Assessment of cognitive and psychomotor impairment, subjective effects, and blood THC concentrations following acute administration of oral and vaporized cannabis. J Psychopharmacol. 2021;35:786–803.
Mayhew DR, Simpson HM, Wood KM, Lonero L, Clinton KM, Johnson AG. On-road and simulated driving: concurrent and discriminant validation. J Saf Res. 2011;42:267–75.
Zamarripa CA, Novak MD, Weerts EM, Vandrey R, Spindle TR. The effects of oral and vaporized cannabis alone, and in combination with alcohol, on driving performance using the STISIM driving simulator: a two-part, double-blind, double-dummy, placebo-controlled, randomized crossover clinical laboratory protocol. Front Pharm. 2022;13:964749.
Sempio C, Almaraz-Quinones N, Jackson M, Zhao W, Wang GS, Liu Y, et al. Simultaneous quantification of 17 cannabinoids by LC–MS-MS in human plasma. J Anal Toxicol. 2022;46:383–92.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300.
Gorbenko AA, Heuberger JA, Klumpers LE, de Kam ML, Strugala PK, de Visser SJ, et al. Cannabidiol increases psychotropic effects and plasma concentrations of Δ9-tetrahydrocannabinol without improving its analgesic properties. Clin Pharm Therapeutics. 2024;116:1289–303.
Chesney E, Oliver D, Sarma A, Lamper AD, Slimani I, Lloyd M, et al. Does cannabidiol reduce the adverse effects of cannabis in schizophrenia? A randomised, double-blind, cross-over trial. Neuropsychopharmacology. 2025; https://doi.org/10.1038/s41386-025-02175-3. Epub ahead of print.
Lo LA, Christiansen AL, Strickland JC, Pistawka CA, Eadie L, Vandrey R, et al. Does acute cannabidiol (CBD) use impair performance? A meta-analysis and comparison with placebo and delta-9-tetrahydrocannabinol (THC). Neuropsychopharmacology. 2024;49:1425–36.
Lo LA, Christiansen A, Eadie L, Strickland JC, Kim DD, Boivin M, et al. Cannabidiol-associated hepatotoxicity: a systematic review and meta-analysis. J Intern Med. 2023;293:724–52.
Marczinski CA, Fillmore MT. Energy drinks mixed with alcohol: what are the risks?. Nutr Rev. 2014;72:98–107.
McKetin R, Coen A, Kaye S. A comprehensive review of the effects of mixing caffeinated energy drinks with alcohol. Drug Alcohol Depend. 2015;151:15–30.
Funding
Support for this study was provided by a research contract from Canopy Growth Corporation and professional development funds from Johns Hopkins University. Canopy Growth Corporation had no direct role in data collection, analysis, or restrictions on publishing of data generated during the conduct of the study.
Author information
Authors and Affiliations
Contributions
JCS, RV, CAZ, TRS, DL, CLB, MTF, JGI, and MOBM contributed to study design. JCS, HET, NMP, and DW contributed to study oversight and data collection. JK, CS, JCP, and UC contributed to pharmacokinetic analysis. JCS and CAZ contributed to data analysis. JCS drafted the initial manuscript with critical feedback from all authors.
Corresponding author
Ethics declarations
Competing interests
JCS has received research related funding from Canopy Growth Corporation and DynamiCare Health and consulting fees from Realm of Caring Foundation and Merck Corporation in the past three years. TRS has received research funding from Cultivate Biologics and consulting fees from Canopy Growth in the past three years. MOBM is employed by Charlotte’s Web, serves as a board member of DeFloria, and is a past employee of Canopy Growth Corporation. The remaining authors have nothing to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Strickland, J.C., Tilton, H.E., Patton, N.M. et al. Effect of caffeine and cannabidiol (CBD) co-administration on Δ9-tetrahydrocannabinol (Δ9-THC) subjective effects, performance impairment, and pharmacokinetics. Neuropsychopharmacol. 50, 1827–1835 (2025). https://doi.org/10.1038/s41386-025-02232-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-025-02232-x