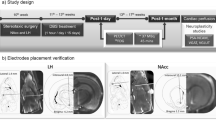
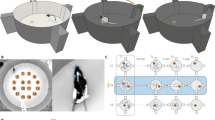
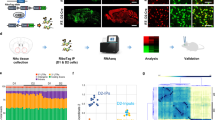
Abstract
Deep brain stimulation (DBS) targeting the nucleus accumbens (NAc) is a promising therapeutic intervention for treatment-resistant neuropsychiatric disorders such as depression, anxiety, and addiction. However, the molecular mechanisms underlying the clinical efficacy of NAc DBS remain largely unknown. One approach to address this question is by performing spatial gene expression analysis on cells located in different regions of the same circuit following NAc DBS. In this study, we utilized high-resolution spatial transcriptomics (Stereo-seq) to investigate gene expression changes induced by NAc DBS in the mouse brain. Mice were randomly allocated to receive continuous electrical stimulation (0.1 mA, 130 Hz) or sham treatment (electrode implanted, no electrical stimulation given) for one week, and subsequent Stereo-seq analysis identified differentially expressed genes (DEGs) across various brain regions. Functional enrichment analysis highlighted changes in synaptic and neuroplasticity processes as well as stress and inflammatory responses in the NAc circuit. Single-cell resolution mapping further identified key molecular players, including Nlgn1, Snca, Pde10a, and Syt1, particularly in glutamate receptor-expressing neurons in the NAc. These genes are critical for synaptic plasticity and neurotransmitter release, and have been implicated in various psychiatric disorders. These findings shed light on the molecular underpinnings of NAc DBS and provide insights into its therapeutic potential in modulating neural circuits associated with neuropsychiatric disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The raw data generated in this study have been deposited to CNGB Nucleotide Sequence Archive with accession number CNP0006277 (https://db.cngb.org/search/project/CNP0006277). The code used to generate the results of this study is available on the GitHub repository at https://github.com/Hemmings-Wu-Lab/NAc-DBS-Transcriptomics.
References
Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, et al. Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell. 2015;162:622–34.
de Jong JW, Afjei SA, Pollak Dorocic I, Peck JR, Liu C, Kim CK, et al. A neural circuit mechanism for encoding aversive stimuli in the mesolimbic dopamine system. Neuron. 2019;101:133–51e7.
Bagot RC, Parise EM, Pena CJ, Zhang HX, Maze I, Chaudhury D, et al. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat Commun. 2015;6:7062.
Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–63.
Milton LK, Mirabella PN, Greaves E, Spanswick DC, van den Buuse M, Oldfield BJ, et al. Suppression of corticostriatal circuit activity improves cognitive flexibility and prevents body weight loss in activity-based anorexia in rats. Biol Psychiatry. 2021;90:819–28.
Zhang Y, Roy DS, Zhu Y, Chen Y, Aida T, Hou Y, et al. Targeting thalamic circuits rescues motor and mood deficits in PD mice. Nature. 2022;607:321–29.
Zhang XY, Peng SY, Shen LP, Zhuang QX, Li B, Xie ST, et al. Targeting presynaptic H3 heteroreceptor in nucleus accumbens to improve anxiety and obsessive-compulsive-like behaviors. Proc Natl Acad Sci USA. 2020;117:32155–64.
Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, et al. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science. 2010;330:1677–81.
Yan H, Shlobin NA, Jung Y, Zhang KK, Warsi N, Kulkarni AV, et al. Nucleus accumbens: a systematic review of neural circuitry and clinical studies in healthy and pathological states. J Neurosurg. 2023;138:337–46.
Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67:110–6.
Ooms P, Mantione M, Figee M, Schuurman PR, van den Munckhof P, Denys D. Deep brain stimulation for obsessive-compulsive disorders: long-term analysis of quality of life. J Neurol Neurosurg Psychiatry. 2014;85:153–8.
Wu H, Van Dyck-Lippens PJ, Santegoeds R, van Kuyck K, Gabriels L, Lin G, et al. Deep-brain stimulation for anorexia nervosa. World Neurosurg. 2013;80:S29 e1–10.
Davidson B, Giacobbe P, George TP, Nestor SM, Rabin JS, Goubran M, et al. Deep brain stimulation of the nucleus accumbens in the treatment of severe alcohol use disorder: a phase I pilot trial. Mol Psychiatry. 2022;27:3992–4000.
Chiken S, Nambu A. High-frequency pallidal stimulation disrupts information flow through the pallidum by GABAergic inhibition. J Neurosci. 2013;33:2268–80.
Herrington TM, Cheng JJ, Eskandar EN. Mechanisms of deep brain stimulation. J Neurophysiol. 2016;115:19–38.
Shi Y, Wang M, Xiao L, Gui L, Zheng W, Bai L, et al. Potential therapeutic mechanism of deep brain stimulation of the nucleus accumbens in obsessive-compulsive disorder. Front Cell Neurosci. 2022;16:1057887.
Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916–23.
Dostrovsky JO, Levy R, Wu JP, Hutchison WD, Tasker RR, Lozano AM. Microstimulation-induced inhibition of neuronal firing in human globus pallidus. J Neurophysiol. 2000;84:570–4.
Shen KZ, Zhu ZT, Munhall A, Johnson SW. Synaptic plasticity in rat subthalamic nucleus induced by high-frequency stimulation. Synapse. 2003;50:314–9.
Creed M, Pascoli VJ, Luscher C. Addiction therapy. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science. 2015;347:659–64.
Bewernick BH, Kayser S, Sturm V, Schlaepfer TE. Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: evidence for sustained efficacy. Neuropsychopharmacology. 2012;37:1975–85.
van Dijk A, Klompmakers AA, Feenstra MG, Denys D. Deep brain stimulation of the accumbens increases dopamine, serotonin, and noradrenaline in the prefrontal cortex. J Neurochem. 2012;123:897–903.
Varatharajan R, Joseph K, Neto SC, Hofmann UG, Moser A, Tronnier V. Electrical high frequency stimulation modulates GABAergic activity in the nucleus accumbens of freely moving rats. Neurochem Int. 2015;90:255–60.
Goodman WK, Storch EA, Sheth SA. Harmonizing the neurobiology and treatment of obsessive-compulsive disorder. Am J Psychiatry. 2021;178:17–29.
Pohodich AE, Yalamanchili H, Raman AT, Wan YW, Gundry M, Hao S, et al. Forniceal deep brain stimulation induces gene expression and splicing changes that promote neurogenesis and plasticity. Elife. 2018;7:e34031.
Faust K, Vajkoczy P, Xi B, Harnack D. The effects of deep brain stimulation of the subthalamic nucleus on vascular endothelial growth factor, brain-derived neurotrophic factor, and glial cell line-derived neurotrophic factor in a rat model of Parkinson’s disease. Stereotact Funct Neurosurg. 2021;99:256–66.
Stahl PL, Salmen F, Vickovic S, Lundmark A, Navarro JF, Magnusson J, et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science. 2016;353:78–82.
Rao A, Barkley D, Franca GS, Yanai I. Exploring tissue architecture using spatial transcriptomics. Nature. 2021;596:211–20.
Wu H, Miller KJ, Blumenfeld Z, Williams NR, Ravikumar VK, Lee KE, et al. Closing the loop on impulsivity via nucleus accumbens delta-band activity in mice and man. Proc Natl Acad Sci USA. 2018;115:192–97.
Zheng Y, Gao H, Zhang J, Wang Y, Zhang S, Xu K in 8th International IEEE/EMBS Conference on Neural Engineering (NER) 259-62 (Shanghai, China, 2017).
Chen A, Liao S, Cheng M, Ma K, Wu L, Lai Y, et al. Spatiotemporal transcriptomic atlas of mouse organogenesis using DNA nanoball-patterned arrays. Cell. 2022;185:1777–92.e21.
Gong C, Li S, Wang L, Zhao F, Fang S, Yuan D, et al. SAW: an efficient and accurate data analysis workflow for Stereo-seq spatial transcriptomics. GigaByte. 2024;2024:gigabyte111.
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21.
van der Walt S, Schönberger JL, Nunez-Iglesias J, Boulogne F, Warner JD, Yager N, et al. scikit-image: image processing in Python. PeerJ. 2014;2:e453.
Fang S, Xu M, Cao L, Liu X, Bezulj M, Tan L, et al. Stereopy: modeling comparative and spatiotemporal cellular heterogeneity via multi-sample spatial transcriptomics. Nat Commun. 2025;16:3741.
Morra JT, Glick SD, Cheer JF. Neural encoding of psychomotor activation in the nucleus accumbens core, but not the shell, requires cannabinoid receptor signaling. J Neurosci. 2010;30:5102–7.
Miszkiel J, Jastrzebska J, Filip M, Przegalinski E. Amphetamine self-administration and its extinction alter the 5-HT(1B) receptor protein levels in designated structures of the rat brain. Neurotox Res. 2019;35:217–29.
Hao Y, Hao S, Andersen-Nissen E, Mauck WM 3rd, Zheng S, Butler A, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573–87.e29.
Korsunsky I, Millard N, Fan J, Slowikowski K, Zhang F, Wei K, et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat Methods. 2019;16:1289–96.
Wu T, Hu E, Xu S, Chen M, Guo P, Dai Z, et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation. 2021;2:100141.
Fetcho RN, Parekh PK, Chou J, Kenwood M, Chalençon L, Estrin DJ, et al. A stress-sensitive frontostriatal circuit supporting effortful reward-seeking behavior. Neuron. 2024;112:473–87.e4.
Gabbay V, Mao X, Klein RG, Ely BA, Babb JS, Panzer AM, et al. Anterior cingulate cortex γ-aminobutyric acid in depressed adolescents: relationship to anhedonia. Arch Gen Psychiatry. 2012;69:139–49.
Gabbay V, Bradley KA, Mao X, Ostrover R, Kang G, Shungu DC. Anterior cingulate cortex γ-aminobutyric acid deficits in youth with depression. Transl Psychiatry. 2017;7:e1216.
Smith KR, Oliver PL, Lumb MJ, Arancibia-Carcamo IL, Revilla-Sanchez R, Brandon NJ, et al. Identification and characterisation of a Maf1/Macoco protein complex that interacts with GABAA receptors in neurons. Mol Cell Neurosci. 2010;44:330–41.
Luykx JJ, Laban KG, van den Heuvel MP, Boks MP, Mandl RC, Kahn RS, et al. Region and state specific glutamate downregulation in major depressive disorder: a meta-analysis of (1)H-MRS findings. Neurosci Biobehav Rev. 2012;36:198–205.
Zhang K, Guo YC, Wang XD, Zhu YJ, Pan BX, Deng C, et al. Lateral septum inputs to nucleus accumbens mediates stress induced suppression of natural reward seeking. Pharm Res. 2022;184:106463.
Yamashita N, Usui H, Nakamura F, Chen S, Sasaki Y, Hida T, et al. Plexin-A4-dependent retrograde semaphorin 3A signalling regulates the dendritic localization of GluA2-containing AMPA receptors. Nat Commun. 2014;5:3424.
Liu J, Yu B, Orozco-Cabal L, Grigoriadis DE, Rivier J, Vale WW, et al. Chronic cocaine administration switches corticotropin-releasing factor2 receptor-mediated depression to facilitation of glutamatergic transmission in the lateral septum. J Neurosci. 2005;25:577–83.
Xu W, Wang J, Li XN, Liang J, Song L, Wu Y, et al. Neuronal and synaptic adaptations underlying the benefits of deep brain stimulation for Parkinson’s disease. Transl Neurodegener. 2023;12:55.
Anderson RW, Farokhniaee A, Gunalan K, Howell B, McIntyre CC. Action potential initiation, propagation, and cortical invasion in the hyperdirect pathway during subthalamic deep brain stimulation. Brain Stimul. 2018;11:1140–50.
Li Q, Ke Y, Chan DC, Qian ZM, Yung KK, Ko H, et al. Therapeutic deep brain stimulation in Parkinsonian rats directly influences motor cortex. Neuron. 2012;76:1030–41.
Kang G, Lowery MM. Effects of antidromic and orthodromic activation of STN afferent axons during DBS in Parkinson’s disease: a simulation study. Front Comput Neurosci. 2014;8:32.
Suppa A, Marsili L, Di Stasio F, Berardelli I, Roselli V, Pasquini M, et al. Cortical and brainstem plasticity in Tourette syndrome and obsessive-compulsive disorder. Mov Disord. 2014;29:1523–31.
Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–58.
Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22:238–49.
Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–57.
Holmes SE, Scheinost D, Finnema SJ, Naganawa M, Davis MT, DellaGioia N, et al. Lower synaptic density is associated with depression severity and network alterations. Nat Commun. 2019;10:1529.
Khambhati AN, Shafi A, Rao VR, Chang EF. Long-term brain network reorganization predicts responsive neurostimulation outcomes for focal epilepsy. Sci Transl Med. 2021;13:eabf6588.
Cooperrider J, Furmaga H, Plow E, Park HJ, Chen Z, Kidd G, et al. Chronic deep cerebellar stimulation promotes long-term potentiation, microstructural plasticity, and reorganization of perilesional cortical representation in a rodent model. J Neurosci. 2014;34:9040–50.
Ruge D, Cif L, Limousin P, Gonzalez V, Vasques X, Hariz MI, et al. Shaping reversibility? Long-term deep brain stimulation in dystonia: the relationship between effects on electrophysiology and clinical symptoms. Brain. 2011;134:2106–15.
Mu Y, Ren Z, Jia J, Gao B, Zheng L, Wang G, et al. Inhibition of phosphodiesterase10A attenuates morphine-induced conditioned place preference. Mol Brain. 2014;7:70.
Mota E, Bompierre S, Betolngar D, Castro LRV, Vincent P. Pivotal role of phosphodiesterase 10A in the integration of dopamine signals in mice striatal D(1) and D(2) medium-sized spiny neurones. Br J Pharm. 2021;178:4873–90.
Gazzellone MJ, Zarrei M, Burton CL, Walker S, Uddin M, Shaheen SM, et al. Uncovering obsessive-compulsive disorder risk genes in a pediatric cohort by high-resolution analysis of copy number variation. J Neurodev Disord. 2016;8:36.
Du T, Li G, Luo H, Pan Y, Xu Q, Ma K. Hippocampal alpha-synuclein mediates depressive-like behaviors. Brain Behav Immun. 2021;95:226–37.
Rotter A, Lenz B, Pitsch R, Richter-Schmidinger T, Kornhuber J, Rhein C. Alpha-synuclein RNA expression is increased in major depression. Int J Mol Sci. 2019;20:2029.
Mazumder S, Bahar AY, Shepherd CE, Prasad AA. Post-mortem brain histological examination in the substantia nigra and subthalamic nucleus in Parkinson’s disease following deep brain stimulation. Front Neurosci. 2022;16:948523.
Pal GD, Ouyang B, Serrano G, Shill HA, Goetz C, Stebbins G, et al. Comparison of neuropathology in Parkinson’s disease subjects with and without deep brain stimulation. Mov Disord. 2017;32:274–77.
Barbier E, Barchiesi R, Domi A, Chanthongdee K, Domi E, Augier G, et al. Downregulation of synaptotagmin 1 in the prelimbic cortex drives alcohol-associated behaviors in rats. Biol Psychiatry. 2021;89:398–406.
Blokland GAM, Grove J, Chen CY, Cotsapas C, Tobet S, Handa R, et al. Sex-dependent shared and nonshared genetic architecture across mood and psychotic disorders. Biol Psychiatry. 2022;91:102–17.
Morozova TV, Goldman D, Mackay TF, Anholt RR. The genetic basis of alcoholism: multiple phenotypes, many genes, complex networks. Genome Biol. 2012;13:239.
Polushina T, Banerjee N, Giddaluru S, Bettella F, Espeseth T, Lundervold AJ, et al. Identification of pleiotropy at the gene level between psychiatric disorders and related traits. Transl Psychiatry. 2021;11:410.
Manville RW, Hogenkamp D, Abbott GW. Ancient medicinal plant rosemary contains a highly efficacious and isoform-selective KCNQ potassium channel opener. Commun Biol. 2023;6:644.
Ali SR, Malone TJ, Zhang Y, Prechova M, Kaczmarek LK. Phactr1 regulates Slack (KCNT1) channels via protein phosphatase 1 (PP1). FASEB J. 2020;34:1591–601.
Zhang Q, Gao SH, Shen ZS, Wang Y, Hu SW, Duan GB, et al. The slack channel regulates anxiety-like behaviors via basolateral amygdala glutamatergic projections to ventral hippocampus. J Neurosci. 2022;42:3049–64.
Mi J, Yang Y, Yao H, Huan Z, Xu C, Ren Z, et al. Inhibition of heat shock protein family A member 8 attenuates spinal cord ischemia-reperfusion injury via astrocyte NF-kappaB/NLRP3 inflammasome pathway : HSPA8 inhibition protects spinal ischemia-reperfusion injury. J Neuroinflammation. 2021;18:170.
Stricher F, Macri C, Ruff M, Muller S. HSPA8/HSC70 chaperone protein: structure, function, and chemical targeting. Autophagy. 2013;9:1937–54.
Wen M, Ma X, Cheng H, Jiang W, Xu X, Zhang Y, et al. Stk38 protein kinase preferentially inhibits TLR9-activated inflammatory responses by promoting MEKK2 ubiquitination in macrophages. Nat Commun. 2015;6:7167.
Vega-Villar M, Horvitz JC, Nicola SM. NMDA receptor-dependent plasticity in the nucleus accumbens connects reward-predictive cues to approach responses. Nat Commun. 2019;10:4429.
Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010;35:185–211.
Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharm. 2004;4:23–9.
Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–80.
McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–7.
Barbano MF, Qi J, Chen E, Mohammad U, Espinoza O, Candido M, et al. VTA glutamatergic projections to the nucleus accumbens suppress psychostimulant-seeking behavior. Neuropsychopharmacology. 2024;49:1905–15.
Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, et al. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22:2916–25.
Yan N, Chen N, Zhu H, Zhang J, Sim M, Ma Y, et al. High-frequency stimulation of nucleus accumbens changes in dopaminergic reward circuit. PLoS One. 2013;8:e79318.
Jimenez-Sanchez L, Linge R, Campa L, Valdizan EM, Pazos A, Diaz A, et al. Behavioral, neurochemical and molecular changes after acute deep brain stimulation of the infralimbic prefrontal cortex. Neuropharmacology. 2016;108:91–102.
Vibholm AK, Landau AM, Alstrup AKO, Jacobsen J, Vang K, Munk OL, et al. Activation of NMDA receptor ion channels by deep brain stimulation in the pig visualised with [(18)F]GE-179 PET. Brain Stimul. 2020;13:1071–78.
Malenka RC, Nicoll RA. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends Neurosci. 1993;16:521–7.
Xu L, Nan J, Lan Y. The nucleus accumbens: a common target in the comorbidity of depression and addiction. Front Neural Circuits. 2020;14:37.
Kim SJ, Lee H, Lee G, Oh SJ, Shin MK, Shim I, et al. CD4+CD25+ regulatory T cell depletion modulates anxiety and depression-like behaviors in mice. PLoS One. 2012;7:e42054.
Rodriguez N, Morer A, Gonzalez-Navarro EA, Serra-Pages C, Boloc D, Torres T, et al. Altered frequencies of Th17 and Treg cells in children and adolescents with obsessive-compulsive disorder. Brain Behav Immun. 2019;81:608–16.
Cromwell HC, Schultz W. Effects of expectations for different reward magnitudes on neuronal activity in primate striatum. J Neurophysiol. 2003;89:2823–38.
Grembecka B, Glac W, Listowska M, Jerzemowska G, Plucinska K, Majkutewicz I, et al. Subthalamic deep brain stimulation affects plasma corticosterone concentration and peripheral immunity changes in rat model of Parkinson’s disease. J Neuroimmune Pharm. 2021;16:454–69.
Basnyat P, Jarvenpaa S, Raitanen J, Pesu M, Lehtimaki K, Peltola J. A 1-year follow-up study on immunological changes following deep brain stimulation in patients with epilepsy. Sci Rep. 2021;11:13765.
Cookson MR. alpha-Synuclein and neuronal cell death. Mol Neurodegener. 2009;4:9.
Tian Y, Lu J, Hao X, Li H, Zhang G, Liu X, et al. FTH1 inhibits ferroptosis through ferritinophagy in the 6-OHDA model of Parkinson’s disease. Neurotherapeutics. 2020;17:1796–812.
Vedam-Mai V, Baradaran-Shoraka M, Reynolds BA, Okun MS. Tissue response to deep brain stimulation and microlesion: a comparative study. Neuromodulation. 2016;19:451–8.
Salatino JW, Ludwig KA, Kozai TDY, Purcell EK. Glial responses to implanted electrodes in the brain. Nat Biomed Eng. 2017;1:862–77.
Hamani C, Davidson B, Lipsman N, Abrahao A, Nestor SM, Rabin JS, et al. Insertional effect following electrode implantation: an underreported but important phenomenon. Brain Commun. 2024;6:fcae093.
Fernandez-Cabrera MR, Selvas A, Miguens M, Higuera-Matas A, Vale-Martinez A, Ambrosio E, et al. Parafascicular thalamic nucleus deep brain stimulation decreases NMDA receptor GluN1 subunit gene expression in the prefrontal cortex. Neuroscience. 2017;348:73–82.
Selvakumar T, Alavian KN, Tierney T. Analysis of gene expression changes in the rat hippocampus after deep brain stimulation of the anterior thalamic nucleus. J Vis Exp. 2015;8:52457.
Acknowledgements
We thank Chang Wang for her contribution in experimental procedure in this study.
Funding
This study was funded by the NSFC Research Grant (82171519) and the NSFC Research Grant (82401781) to ZZ.
Author information
Authors and Affiliations
Contributions
CC, HW, and JZ conceived and designed the project. CC, LG, and HW contributed to the analysis and interpretation of data and wrote the manuscript. CC and ZZ contributed to the data acquisition, statistical analyses, and prepared the tables and figures. LG, WC, CY, and KX verified the results. LG, HW, and JZ edited the manuscript and provided supervision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All animal procedures were approved by the Second Affiliated Hospital of Zhejiang University—Animal Ethics Committee (Approval No. AIRB-2021-087) and were performed according to the Guiding Principles for the Care and Use of Laboratory Animals Approved by Animal Regulations of National Science and Technology Committee of China.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cai, C., Gao, L., Zhu, Z. et al. Change in brain molecular landscapes following electrical stimulation of the nucleus accumbens. Neuropsychopharmacol. 51, 464–475 (2026). https://doi.org/10.1038/s41386-025-02241-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-025-02241-w