Abstract
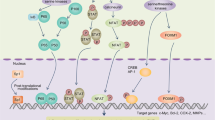
Colorectal cancer (CRC) is the most common digestive tract malignancy, attributing to approximately 9.4% of global cancer-related deaths. However, the pathogenesis of CRC is poorly understood. The testis-expressed 11 (TEX11) gene is located on the X chromosome and is required for spermatogenesis, and is reported might serve as a biomarker for early onset CRC according to database analysis. However, the role played by TEX11 in cancer progression remains to be investigated. In this study, we show that TEX11 expression is significantly downregulated in CRC cell lines and clinical CRC tissue samples, and TEX11 expression correlates with poor prognosis in CRC patients. We further demonstrate that TEX11 can significantly inhibit the proliferative capacity of CRC cells in vitro and in vivo. Mechanistically, we demonstrate that TEX11 promotes transcription of COP1 by upregulating FOXO3a expression. This enhanced COP1 expression subsequently accelerates the degradation of the negative transcriptional regulator c-Jun, which, in turn, enhances p21 transcription inhibiting CRC cell cycle progression and proliferation. Overall, our findings suggest that TEX11 may be a valuable therapeutic target for the treatment of CRC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
Data availability
The dataset used during the study is available from the corresponding author on reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49.
Boeding JRE, Ramphal W, Crolla R, Boonman-de Winter LJM, Gobardhan PD, Schreinemakers JMJ. Ileus caused by obstructing colorectal cancer-impact on long-term survival. Int J Colorectal Dis. 2018;33:1393–400.
Akgul O, Cetinkaya E, Ersoz S, Tez M. Role of surgery in colorectal cancer liver metastases. World J Gastroenterol. 2014;20:6113–22.
Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther. 2020;5:22.
Marmol I, Sanchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int J Mol Sci. 2017;18:197.
Garciaa R, Aguilera A, Contreras-Esquivel JC, Rodriguez R, Aguilar CN. Extraction of condensed tannins from Mexican plant sources. Z Naturforsch C J Biosci. 2008;63:17–20.
Tuttelmann F, Ruckert C, Ropke A. Disorders of spermatogenesis: Perspectives for novel genetic diagnostics after 20 years of unchanged routine. Med Genet. 2018;30:12–20.
Guiraldelli MF, Felberg A, Almeida LP, Parikh A, de Castro RO, Pezza RJ. SHOC1 is a ERCC4-(HhH)2-like protein, integral to the formation of crossover recombination intermediates during mammalian meiosis. PLoS Genet. 2018;14:e1007381.
Dapper AL, Payseur BA. Molecular evolution of the meiotic recombination pathway in mammals. Evolution. 2019;73:2368–89.
Luo T, Wu S, Shen X, Li L. Network cluster analysis of protein-protein interaction network identified biomarker for early onset colorectal cancer. Mol Biol Rep. 2013;40:6561–8.
Zhang Y, Chen F, Chandrashekar DS, Varambally S, Creighton CJ. Proteogenomic characterization of 2002 human cancers reveals pan-cancer molecular subtypes and associated pathways. Nat Commun. 2022;13:2669.
Chen F, Chandrashekar DS, Scheurer ME, Varambally S, Creighton CJ. Global molecular alterations involving recurrence or progression of pediatric brain tumors. Neoplasia. 2022;24:22–33.
Blanchard JM. Cyclin A2 transcriptional regulation: modulation of cell cycle control at the G1/S transition by peripheral cues. Biochem Pharm. 2000;60:1179–84.
Hume S, Dianov GL, Ramadan K. A unified model for the G1/S cell cycle transition. Nucl. Acids Res. 2020;48:12483–501.
El-Deiry WS. p21(WAF1) Mediates Cell-Cycle Inhibition, Relevant to Cancer Suppression and Therapy. Cancer Res. 2016;76:5189–91.
Messeguer X, Escudero R, Farre D, Nunez O, Martinez J, Alba MM. PROMO: detection of known transcription regulatory elements using species-tailored searches. Bioinformatics. 2002;18:333–4.
Farre D, Roset R, Huerta M, Adsuara JE, Rosello L, Alba MM, et al. Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Res. 2003;31:3651–3.
Fornes O, Castro-Mondragon JA, Khan A, van der Lee R, Zhang X, Richmond PA, et al. JASPAR 2020: update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2020;48:D87–D92.
Wang CH, Tsao YP, Chen HJ, Chen HL, Wang HW, Chen SL. Transcriptional repression of p21((Waf1/Cip1/Sdi1)) gene by c-jun through Sp1 site. Biochem Biophys Res Commun. 2000;270:303–10.
Cheng JM, Yao MR, Zhu Q, Wu XY, Zhou J, Tan WL, et al. Silencing of stat4 gene inhibits cell proliferation and invasion of colorectal cancer cells. J Biol Regul Homeost Agents. 2015;29:85–92.
Hershko A. The ubiquitin pathway for protein degradation. Trends Biochem Sci. 1991;16:265–8.
Wang Y, Le WD. Autophagy and Ubiquitin-Proteasome System. Adv Exp Med Biol. 2019;1206:527–50.
Wang ZB, Liu YQ, Cui YF. Pathways to caspase activation. Cell Biol Int. 2005;29:489–96.
Csizmok V, Montecchio M, Lin H, Tyers M, Sunnerhagen M, Forman-Kay JD. Multivalent Interactions with Fbw7 and Pin1 Facilitate Recognition of c-Jun by the SCF(Fbw7) Ubiquitin Ligase. Structure. 2018;26:28–39.e22.
Migliorini D, Bogaerts S, Defever D, Vyas R, Denecker G, Radaelli E, et al. Cop1 constitutively regulates c-Jun protein stability and functions as a tumor suppressor in mice. J Clin Invest. 2011;121:1329–43.
Gao B, Lee SM, Fang D. The tyrosine kinase c-Abl protects c-Jun from ubiquitination-mediated degradation in T cells. J Biol Chem. 2006;281:29711–8.
Yang F, Silber S, Leu NA, Oates RD, Marszalek JD, Skaletsky H, et al. TEX11 is mutated in infertile men with azoospermia and regulates genome-wide recombination rates in mouse. EMBO Mol Med. 2015;7:1198–210.
Yatsenko AN, Georgiadis AP, Ropke A, Berman AJ, Jaffe T, Olszewska M, et al. X-linked TEX11 mutations, meiotic arrest, and azoospermia in infertile men. N Engl J Med. 2015;372:2097–107.
Zheng K, Yang F, Wang PJ. Regulation of male fertility by X-linked genes. J Androl. 2010;31:79–85.
Karimian A, Ahmadi Y, Yousefi B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair (Amst). 2016;42:63–71.
Shamloo B. Usluer S p21 in Cancer Research. Cancers (Basel). 2019;11:1178.
Song Y, Liu Y, Pan S, Xie S, Wang ZW, Zhu X. Role of the COP1 protein in cancer development and therapy. Semin Cancer Biol. 2020;67:43–52.
Fasano C, Disciglio V, Bertora S, Lepore Signorile M, Simone C. FOXO3a from the Nucleus to the Mitochondria: A Round Trip in Cellular Stress Response. Cells. 2019;8:1110.
Nho RS, Hergert P. FoxO3a and disease progression. World J Biol Chem. 2014;5:346–54.
Wang F, Marshall CB, Ikura M. Forkhead followed by disordered tail: The intrinsically disordered regions of FOXO3a. Intrinsically Disord Proteins. 2015;3:e1056906.
Nestal de Moraes G, Bella L, Zona S, Burton MJ, Lam EW. Insights into a Critical Role of the FOXO3a-FOXM1 Axis in DNA Damage Response and Genotoxic Drug Resistance. Curr Drug Targets. 2016;17:164–77.
Liu Y, Ao X, Ding W, Ponnusamy M, Wu W, Hao X, et al. Critical role of FOXO3a in carcinogenesis. Mol Cancer. 2018;17:104.
Chacko L, Macaron C, Burke CA. Colorectal cancer screening and prevention in women. Dig Dis Sci. 2015;60:698–710.
Koo JH, Leong RW. Sex differences in epidemiological, clinical and pathological characteristics of colorectal cancer. J Gastroenterol Hepatol. 2010;25:33–42.
Ray AL, Nofchissey RA, Khan MA, Reidy MA, Lerner MR, Wu X, et al. The role of sex in the innate and adaptive immune environment of metastatic colorectal cancer. Br J Cancer. 2020;123:624–32.
Gausman V, Dornblaser D, Anand S, Hayes RB, O’Connell K, Du M, et al. Risk Factors Associated With Early-Onset Colorectal Cancer. Clin Gastroenterol Hepatol. 2020;18:2752–9.e2752.
Lukashev AN, Zamyatnin AA Jr. Viral Vectors for Gene Therapy: Current State and Clinical Perspectives. Biochem (Mosc). 2016;81:700–8.
Watanabe M, Nishikawaji Y, Kawakami H, Kosai KI. Adenovirus Biology, Recombinant Adenovirus, and Adenovirus Usage in Gene Therapy. Viruses. 2021;13:2502.
Li H, Zhang N, Jiao X, Wang C, Sun W, He Y, et al. Downregulation of microRNA-6125 promotes colorectal cancer growth through YTHDF2-dependent recognition of N6-methyladenosine-modified GSK3beta. Clin Transl Med. 2021;11:e602.
Jin H, Sun W, Zhang Y, Yan H, Liufu H, Wang S, et al. MicroRNA-411 Downregulation Enhances Tumor Growth by Upregulating MLLT11 Expression in Human Bladder Cancer. Mol Ther Nucleic Acids. 2018;11:312–22.
Acknowledgements
Bioinformatics results described here are based on data from the TCGA research network (http://cancergenome.nih.gov/). We thank all participants, specimen donors, and research groups who participated in the creation of the TCGA CRC cancer dataset. This work was partially supported by the Key Project of Science and Technology Innovation Team of Zhejiang Province (2013TD10), Key Discipline of Zhejiang Province in Medical Technology (First Class, Category A), Wenzhou Science & Technology Bureau (Y20180857), and Wenzhou Medical University (89219018).
Author information
Authors and Affiliations
Contributions
LZ, HL, XZ, and YP conceived and designed the study. XZ, XJ, FH, YL, SW, BZ, and GR detected the biological functions of cells, performed the RT-qPCR assays, and conducted statistical analyses. JL and LZ performed animal experiments. XZ and QX collected and analyzed clinical samples. HL, LZ, and XZ drafted the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All experiments related to clinical specimens were approved by the ethics committee of Wenzhou Medical University (YS2019-170). All animal experiments were performed in accordance with the management regulations of the Experimental Animal Ethics Committee of Wenzhou Medical University (WYYY-AEC-YS-2022-0260).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., Hu, F., Zhu, B. et al. Downregulation of TEX11 promotes S-Phase progression and proliferation in colorectal cancer cells through the FOXO3a/COP1/c-Jun/p21 axis. Oncogene 41, 5133–5145 (2022). https://doi.org/10.1038/s41388-022-02490-9
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41388-022-02490-9
This article is cited by
-
Gambogic acid suppresses osteosarcoma progression through upregulation of FOXO3a
Discover Oncology (2025)
-
Tumor-secreted LCN2 impairs gastric cancer progression via autocrine inhibition of the 24p3R/JNK/c-Jun/SPARC axis
Cell Death & Disease (2024)