Abstract
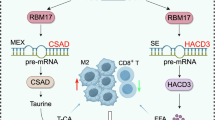
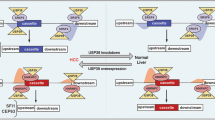
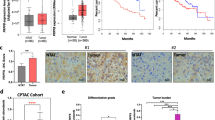
Alternative splicing (AS) is crucial for tumor cells as it regulates protein expression and produces various protein isoforms, which can have diverse or even opposing roles in tumor growth and metastasis. Despite its significance, the role of AS and related splicing factors, particularly splicing-related messenger ribonucleoproteins (mRNPs), in hepatocarcinogenesis, is poorly understood. High-throughput transcriptome sequencing of HCC patients revealed that the spliceosome pathway might play a significant role in HCC development. Through the combined analysis of the three gene clusters, the splicing factor RBM39 was identified, which was highly expressed in HCC tumor tissues with prognostic value. Functional studies showed that silencing RBM39 inhibited cell proliferation, migration, and invasion via the integrin pathway. By performing RNA immunoprecipitation sequencing (RIP-seq), we found that RBM39 combined to RFX1 pre-mRNA and regulated alternative splicing of exon 2. Mechanistically, the exon 2 skipping in RFX1, influenced by high RBM39 expression in HCC cells, led to the production of an N-terminal truncated RFX1, which lost the transcriptional repression ability on oncogenic collagen genes. High RBM39 expression enhances the malignant capabilities of HCC cells by regulating the alternative splicing of RFX1 and subsequently activating the FAK/PI3K/AKT signaling pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and the Supplementary Materials. The data underlying this article are available in the Gene Expression Omnibus, and can be accessed under accession codes GSE275347 and GSE275496.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2021;71:209–49.
Rahman MA, Krainer AR, Abdel-Wahab O. SnapShot: splicing alterations in cancer. Cell. 2020;180:208–e1.
Bradley RK, Anczuków O. RNA splicing dysregulation and the hallmarks of cancer. Nat Rev Cancer. 2023;23:135–55.
Bonnal SC, López-Oreja I, Valcárcel J. Roles and mechanisms of alternative splicing in cancer - implications for care. Nat Rev Clin Oncol. 2020;17:457–74.
Wang E, Lu SX, Pastore A, Chen X, Imig J, Chun-Wei Lee S, et al. Targeting an RNA-binding protein network in acute myeloid leukemia. Cancer cell. 2019;35:369–84.e7.
Corsini L, Bonnal S, Basquin J, Hothorn M, Scheffzek K, Valcárcel J, et al. U2AF-homology motif interactions are required for alternative splicing regulation by SPF45. Nat Struct Mol Biol. 2007;14:620–9.
Wan L, Yu W, Shen E, Sun W, Liu Y, Kong J, et al. SRSF6-regulated alternative splicing that promotes tumour progression offers a therapy target for colorectal cancer. Gut. 2019;68:118–29.
Jbara A, Lin KT, Stossel C, Siegfried Z, Shqerat H, Amar-Schwartz A, et al. RBFOX2 modulates a metastatic signature of alternative splicing in pancreatic cancer. Nature. 2023;617:147–53.
Wang JZ, Fu X, Fang Z, Liu H, Zong FY, Zhu H, et al. QKI-5 regulates the alternative splicing of cytoskeletal gene ADD3 in lung cancer. J Mol cell Biol. 2021;13:347–60.
Brown RL, Reinke LM, Damerow MS, Perez D, Chodosh LA, Yang J, et al. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J Clin Investig. 2011;121:1064–74.
Qin H, Ni H, Liu Y, Yuan Y, Xi T, Li X, et al. RNA-binding proteins in tumor progression. J Hematol Oncol. 2020;13:90.
Gerstberger S, Hafner M, Tuschl T. A census of human RNA-binding proteins. Nat Rev Genet. 2014;15:829–45.
Sciarrillo R, Wojtuszkiewicz A, Assaraf YG, Jansen G, Kaspers GJL, Giovannetti E, et al. The role of alternative splicing in cancer: From oncogenesis to drug resistance. Drug Resist Updates: Rev Comment Antimicrob Anticancer Chemother. 2020;53:100728.
Xiao Y, Cai GP, Feng X, Li YJ, Guo WH, Guo Q, et al. Splicing factor YBX1 regulates bone marrow stromal cell fate during aging. EMBO J. 2023;42:e111762.
Weber AI, Parthasarathy S, Borisova E, Epifanova E, Preußner M, Rusanova A, et al. Srsf1 and Elavl1 act antagonistically on neuronal fate choice in the developing neocortex by controlling TrkC receptor isoform expression. Nucleic acids Res. 2023;51:10218–37.
Baejen C, Torkler P, Gressel S, Essig K, Söding J, Cramer P. Transcriptome maps of mRNP biogenesis factors define pre-mRNA recognition. Mol cell. 2014;55:745–57.
Liu X, Zheng Y, Xiao M, Chen X, Zhu Y, Xu C, et al. SRSF10 stabilizes CDC25A by triggering exon 6 skipping to promote hepatocarcinogenesis. J Exp Clin Cancer Res. 2022;41:353.
Luo C, Cheng Y, Liu Y, Chen L, Liu L, Wei N, et al. SRSF2 regulates alternative splicing to drive hepatocellular carcinoma development. Cancer Res. 2017;77:1168–78.
Wu Y, Wang J, Zhao T, Sun M, Xu M, Che S, et al. Polystyrenenanoplastics lead to ferroptosis in the lungs. J Adv Res. 2024;56:31–41.
Dowhan DH, Hong EP, Auboeuf D, Dennis AP, Wilson MM, Berget SM, et al. Steroid hormone receptor coactivation and alternative RNA splicing by U2AF65-related proteins CAPERalpha and CAPERbeta. Mol cell. 2005;17:429–39.
Tari M, Manceau V, de Matha Salone J, Kobayashi A, Pastré D, Maucuer A. U2AF(65) assemblies drive sequence-specific splice site recognition. EMBO Rep. 2019;20:e47604.
Han T, Goralski M, Gaskill N, Capota E, Kim J, Ting TC, et al. Anticancer sulfonamides target splicing by inducing RBM39 degradation via recruitment to DCAF15. Science (New York, NY). 2017; 356.
Mai S, Qu X, Li P, Ma Q, Cao C, Liu X. Global regulation of alternative RNA splicing by the SR-rich protein RBM39. Biochimica et Biophys acta. 2016;1859:1014–24.
Mossmann D, Müller C, Park S, Ryback B, Colombi M, Ritter N, et al. Arginine reprograms metabolism in liver cancer via RBM39. Cell. 2023;186:5068–83.e23.
Kang YK, Putluri N, Maity S, Tsimelzon A, Ilkayeva O, Mo Q, et al. CAPER is vital for energy and redox homeostasis by integrating glucose-induced mitochondrial functions via ERR-α-Gabpa and stress-induced adaptive responses via NF-κB-cMYC. PLoS Genet. 2015;11:e1005116.
Yokoi A, Kuromitsu J, Kawai T, Nagasu T, Sugi NH, Yoshimatsu K, et al. Profiling novel sulfonamide antitumor agents with cell-based phenotypic screens and array-based gene expression analysis. Mol Cancer Ther. 2002;1:275–86.
Huang G, Zhou Z, Wang H, Kleinerman ES. CAPER-α alternative splicing regulates the expression of vascular endothelial growth factor165 in Ewing sarcoma cells. Cancer. 2012;118:2106–16.
Lu SX, De Neef E, Thomas JD, Sabio E, Rousseau B, Gigoux M, et al. Pharmacologic modulation of RNA splicing enhances anti-tumor immunity. Cell. 2021;184:4032–47.e31.
Xu Y, Nijhuis A, Keun HC. RNA-binding motif protein 39 (RBM39): An emerging cancer target. Br J Pharmacol. 2022;179:2795–812.
Subramanian P, Gargani S, Palladini A, Chatzimike M, Grzybek M, Peitzsch M, et al. The RNA binding protein human antigen R is a gatekeeper of liver homeostasis. Hepatology. 2022;75:881–97.
Cao C, Sun J, Zhang D, Guo X, Xie L, Li X, et al. The long intergenic noncoding RNA UFC1, a target of MicroRNA 34a, interacts with the mRNA stabilizing protein HuR to increase levels of β-catenin in HCC cells. Gastroenterology. 2015;148:415–26.e18.
Lin Y, Liang R, Qiu Y, Lv Y, Zhang J, Qin G, et al. Expression and gene regulation network of RBM8A in hepatocellular carcinoma based on data mining. Aging. 2019;11:423–47.
Chamberlain PP, Hamann LG. Development of targeted protein degradation therapeutics. Nat Chem Biol. 2019;15:937–44.
Issac J, Raveendran PS, Das AV. RFX1: a promising therapeutic arsenal against cancer. Cancer Cell Int. 2021;21:253.
Liu P, Ge M, Hu J, Li X, Che L, Sun K, et al. A functional mammalian target of rapamycin complex 1 signaling is indispensable for c-Myc-driven hepatocarcinogenesis. Hepatology. 2017;66:167–81.
Lee SE, Alcedo KP, Kim HJ, Snider NT. Alternative splicing in hepatocellular carcinoma. Cell Mol Gastroenterol Hepatol. 2020;10:699–712.
Yu L, Kim J, Jiang L, Feng B, Ying Y, Ji KY, et al. MTR4 drives liver tumorigenesis by promoting cancer metabolic switch through alternative splicing. Nat Commun. 2020;11:708.
Zhou HZ, Li F, Cheng ST, Xu Y, Deng HJ, Gu DY, et al. DDX17-regulated alternative splicing that produced an oncogenic isoform of PXN-AS1 to promote HCC metastasis. Hepatology. 2022;75:847–65.
Královicová J, Ševcíková I, Stejskalová E, Obuca M, Hiller M, Stanek D, et al. PUF60-activated exons uncover altered 3’ splice-site selection by germline missense mutations in a single RRM. Nucleic Acids Res. 2018;46:6166–87.
Desert R, Chen W, Ge X, Viel R, Han H, Athavale D, et al. Hepatocellular carcinomas, exhibiting intratumor fibrosis, express cancer-specific extracellular matrix remodeling and WNT/TGFB signatures, associated with poor outcome. Hepatology. 2023;78:741–57.
Chen Y, Yang S, Tavormina J, Tampe D, Zeisberg M, Wang H, et al. Oncogenic collagen I homotrimers from cancer cells bind to α3β1 integrin and impact tumor microbiome and immunity to promote pancreatic cancer. Cancer Cell. 2022;40:818–34.e9.
Di Martino JS, Nobre AR, Mondal C, Taha I, Farias EF, Fertig EJ, et al. A tumor-derived type III collagen-rich ECM niche regulates tumor cell dormancy. Nat Cancer. 2022;3:90–107.
Omar R, Cooper A, Maranyane HM, Zerbini L, Prince S. COL1A2 is a TBX3 target that mediates its impact on fibrosarcoma and chondrosarcoma cell migration. Cancer Lett. 2019;459:227–39.
Wu X, Cai J, Zuo Z, Li J. Collagen facilitates the colorectal cancer stemness and metastasis through an integrin/PI3K/AKT/Snail signaling pathway. Biomed Pharmacother. 2019;114:108708.
Wang L, Gao Y, Zhao X, Guo C, Wang X, Yang Y, et al. HOXD3 was negatively regulated by YY1 recruiting HDAC1 to suppress progression of hepatocellular carcinoma cells via ITGA2 pathway. Cell Prolif. 2020;53:e12835.
Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer. 2018;18:533–48.
Kubow KE, Vukmirovic R, Zhe L, Klotzsch E, Smith ML, Gourdon D, et al. Mechanical forces regulate the interactions of fibronectin and collagen I in extracellular matrix. Nat Commun. 2015;6:8026.
Pang X, He X, Qiu Z, Zhang H, Xie R, Liu Z, et al. Targeting integrin pathways: mechanisms and advances in therapy. Signal Transduct Target Ther. 2023;8:1.
Li M, Wang Y, Li M, Wu X, Setrerrahmane S, Xu H. Integrins as attractive targets for cancer therapeutics. Acta Pharm Sin B. 2021;11:2726–37.
Wang KR, Nemoto T, Yokota Y. RFX1 mediates the serum-induced immediate early response of Id2 gene expression. J Biol Chem. 2007;282:26167–77.
Jia W, Liang S, Lin W, Li S, Yuan J, Jin M, et al. Hypoxia-induced exosomes facilitate lung pre-metastatic niche formation in hepatocellular carcinoma through the miR-4508-RFX1-IL17A-p38 MAPK-NF-κB pathway. Int J Biol Sci. 2023;19:4744–62.
Liu Y, Jiang P, Wang G, Liu X, Luo S. Downregulation of RFX1 predicts poor prognosis of patients with small hepatocellular carcinoma. Eur J Surg Oncol. 2018;44:1087–93.
Sengupta P, Xu Y, Wang L, Widom R, Smith BD. Collagen alpha1(I) gene (COL1A1) is repressed by RFX family. J Biol Chem. 2005;280:21004–14.
Reith W, Herrero-Sanchez C, Kobr M, Silacci P, Berte C, Barras E, et al. MHC class II regulatory factor RFX has a novel DNA-binding domain and a functionally independent dimerization domain. Genes Dev. 1990;4:1528–40.
Du P, Gao K, Cao Y, Yang S, Wang Y, Guo R, et al. RFX1 downregulation contributes to TLR4 overexpression in CD14(+) monocytes via epigenetic mechanisms in coronary artery disease. Clin Epigenet. 2019;11:44.
Zhao M, Tan Y, Peng Q, Huang C, Guo Y, Liang G, et al. IL-6/STAT3 pathway induced deficiency of RFX1 contributes to Th17-dependent autoimmune diseases via epigenetic regulation. Nat Commun. 2018;9:583.
Tward AD, Jones KD, Yant S, Cheung ST, Fan ST, Chen X, et al. Distinct pathways of genomic progression to benign and malignant tumors of the liver. Proc Natl Acad Sci USA. 2007;104:14771–6.
Chen X, Calvisi DF. Hydrodynamic transfection for generation of novel mouse models for liver cancer research. Am J Pathol. 2014;184:912–23.
Zhou Y, Chen Y, Zhang X, Xu Q, Wu Z, Cao X, et al. Brahma-Related Gene 1 Inhibition Prevents Liver Fibrosis and Cholangiocarcinoma by Attenuating Progenitor Expansion. Hepatology. 2021;74:797–815.
Sebaugh JL. Guidelines for accurate EC50/IC50 estimation. Pharm Stat. 2011;10:128–34.
Rio DC. Electrophoretic mobility shift assays for RNA-protein complexes. Cold Spring Harb Protoc. 2014;2014:435–40.
Gao Q, Zhu H, Dong L, Shi W, Chen R, Song Z, et al. Integrated proteogenomic characterization of HBV-related hepatocellular carcinoma. Cell. 2019;179:1240.
Acknowledgements
This work was supported by grants from the National Key Research and Development Program of China (2023YFC2505900 & 2022YFC2304705), the Natural Science Foundation of China (82173255, 82273330, 82270691), 1.3.5 project for disciplines of excellence from West China Hospital of Sichuan University (No. ZYGD24002), the Key R&D projects of Sichuan Provincial Department of Science and Technology (23ZDYF2182, 23ZDYF2083). We thank http://www.bioinformatics.com.cn/ for plotting part of pictures. We acknowledge the support provided by LC-Bio Technologies (Hangzhou) Co., Ltd. with the RNA sequencing and data analysis. We acknowledge the support provided by SEQHEALTH (Wuhan) Co., Ltd. with the RIP sequencing and data analysis.
Author information
Authors and Affiliations
Contributions
B.Z., X.X., Q.W.Z., and Z.R.W.: performed the experiments and generated data. B.Z., X.X., Y.J.Z., and J.Y.Y.: analyzed the data. B.Z., Y.J.Z., G.X., T.L., and J.Y.Y.: designed the experiments. B.Z. and Y.J.Z.: wrote the manuscript. G.X., Q.W., T.L., and J.Y.: provided clinical samples. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The authors confirm that all methods were performed in accordance with the relevant guidelines and regulations. All animal experiments were approved by the Experimental Animal Care Committee of West China Hospital of Sichuan University (approval number: 20230228051). The use of patient specimens and the relevant database were approved by the Research Ethics Committee of West China Hospital of Sichuan University (approval number: 2020-166).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, B., Zhou, Y., Xu, X. et al. RBM39 promotes hepatocarcinogenesis by regulating RFX1’s alternative splicing and subsequent activation of integrin signaling pathway. Oncogene 44, 1488–1503 (2025). https://doi.org/10.1038/s41388-025-03327-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41388-025-03327-x