Abstract
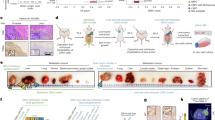
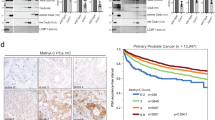
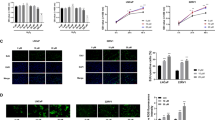
Prostate cancer (PCa) is an androgen-dependent malignancy, with HSP90 and HSP70 serving as classical molecular chaperones that maintain androgen receptor (AR) protein stability and regulate its transcriptional activation. Surprisingly, our study identified TOMM20, a mitochondrial outer membrane protein, as a potential molecular chaperone with similar roles to HSP90/HSP70. We found that TOMM20 expression is elevated in PCa tissues and cell lines and positively correlates with AR levels. RNA-seq analysis revealed that TOMM20 knockdown significantly reduced the mRNA levels of AR-regulated genes. Additionally, the protein level of KLK3 (PSA) decreased, and AR binding to the androgen response element (ARE) of the KLK3 promoter was diminished following TOMM20 knockdown, leading to decreased KLK3 gene transcription. Furthermore, TOMM20 depletion reduced both cytoplasmic and nuclear AR protein levels and facilitated AR degradation via an E3 ubiquitin ligase SKP2-mediated ubiquitin-proteasome pathway, independent of heat shock proteins (HSPs). To our knowledge, this is the first report demonstrating that TOMM20, a mitochondrial outer translocase protein, stabilizes AR protein and enhances its transcriptional activity, while its knockdown promotes AR degradation through the SKP2-mediated ubiquitin-proteasome pathway. These findings suggest that TOMM20 may serve as a potential biomarker for PCa progression and a promising therapeutic target for drug development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The RNA-seq data in this manuscript have been submitted to the National Centre for Biotechnology (BioProject ID:PRJAN783133). The public datasets used in this study were downloaded from the Gene Expression Omnibus database (https://www.ncbi.nlm.nih.gov/geo/) and the cBioPortal(http://www.cbioportal.org/). All relevant data are available from the corresponding authors upon reasonable request.
References
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63.
Aurilio G, Cimadamore A, Mazzucchelli R, Lopez-Beltran A, Verri E, Scarpelli M, et al. Androgen receptor signaling pathway in prostate cancer: from genetics to clinical applications. Cells 2020;9:2653.
Wilding G. The importance of steroid hormones in prostate cancer. Cancer Surv. 1992;14:113–30.
Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–9.
Chang C, Saltzman A, Yeh S, Young W, Keller E, Lee HJ, et al. Androgen receptor: an overview. Crit Rev Eukaryot Gene Expr. 1995;5:97–125.
Fang Y, Fliss AE, Robins DM, Caplan AJ. Hsp90 regulates androgen receptor hormone binding affinity in vivo. J Biol Chem. 1996;271:28697–702.
Dong J, Wu Z, Wang D, Pascal LE, Nelson JB, Wipf P, et al. Hsp70 binds to the androgen receptor N-terminal domain and modulates the receptor function in prostate cancer cells. Mol Cancer Ther. 2019;18:39–50.
Georget V, Terouanne B, Nicolas JC, Sultan C. Mechanism of antiandrogen action: key role of hsp90 in conformational change and transcriptional activity of the androgen receptor. Biochemistry. 2002;41:11824–31.
Prescott J, Coetzee GA. Molecular chaperones throughout the life cycle of the androgen receptor. Cancer Lett. 2006;231:12–19.
Davey RA, Grossmann M. Androgen receptor structure, function and biology: from bench to bedside. Clin Biochem Rev. 2016;37:3–15.
Azad AA, Zoubeidi A, Gleave ME, Chi KN. Targeting heat shock proteins in metastatic castration-resistant prostate cancer. Nat Rev Urol. 2015;12:26–36.
Grupp K, Jedrzejewska K, Tsourlakis MC, Koop C, Wilczak W, Adam M, et al. High mitochondria content is associated with prostate cancer disease progression. Mol Cancer. 2013;12:145.
Masciangelo R, Chiti MC, Camboni A, Amorim CA, Donnez J, Dolmans MM. Mitochondrial content, activity, and morphology in prepubertal and adult human ovaries. J Assist Reprod Genet. 2021;38:2581–90.
Sayyed UMH, Mahalakshmi R. Mitochondrial protein translocation machinery: From TOM structural biogenesis to functional regulation. J Biol Chem. 2022;298:101870.
Zhao Z, Han F, He Y, Yang S, Hua L, Wu J, et al. Stromal-epithelial metabolic coupling in gastric cancer: stromal MCT4 and mitochondrial TOMM20 as poor prognostic factors. Eur J Surg Oncol. 2014;40:1361–8.
Park SH, Lee AR, Choi K, Joung S, Yoon JB, Kim S. TOMM20 as a potential therapeutic target of colorectal cancer. BMB Rep. 2019;52:712–7.
Roche ME, Lin Z, Whitaker-Menezes D, Zhan T, Szuhai K, Bovee J, et al. Translocase of the outer mitochondrial membrane complex subunit 20 (TOMM20) facilitates cancer aggressiveness and therapeutic resistance in chondrosarcoma. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165962.
Yang X, Song D, Zhang J, Yang X, Feng H, Guo J. PRR34-AS1 sponges miR-498 to facilitate TOMM20 and ITGA6 mediated tumor progression in HCC. Exp Mol Pathol. 2021;120:104620.
Levesque MH, El-Alfy M, Cusan L, Labrie F. Androgen receptor as a potential sign of prostate cancer metastasis. Prostate. 2009;69:1704–11.
Yin L, Ye Y, Zou L, Lin J, Dai Y, Fu Y, et al. AR antagonists develop drug resistance through TOMM20 autophagic degradation-promoted transformation to neuroendocrine prostate cancer. J Exp Clin Cancer Res. 2023;42:204.
Li B, Lu W, Chen Z. Regulation of androgen receptor by E3 ubiquitin ligases: for more or less. Receptors Clin Investig. 2014;1:10.
Ratajczak W, Lubkowski M, Lubkowska A. Heat shock proteins in benign prostatic hyperplasia and prostate cancer. Int J Mol Sci. 2022;23:897.
Eftekharzadeh B, Banduseela VC, Chiesa G, Martinez-Cristobal P, Rauch JN, Nath SR, et al. Hsp70 and Hsp40 inhibit an inter-domain interaction necessary for transcriptional activity in the androgen receptor. Nat Commun. 2019;10:3562.
Schleiff E, Turnbull JL. Characterization of the N-terminal targeting signal binding domain of the mitochondrial outer membrane receptor, Tom20. Biochemistry. 1998;37:13052–8.
Zhou B, Zhang JY, Liu XS, Chen HZ, Ai YL, Cheng K, et al. Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis. Cell Res. 2018;28:1171–85.
Lalier L, Mignard V, Joalland MP, Lanoe D, Cartron PF, Manon S, et al. TOM20-mediated transfer of Bcl2 from ER to MAM and mitochondria upon induction of apoptosis. Cell Death Dis. 2021;12:182.
Fu ZJ, Wang ZY, Xu L, Chen XH, Li XX, Liao WT, et al. HIF-1alpha-BNIP3-mediated mitophagy in tubular cells protects against renal ischemia/reperfusion injury. Redox Biol. 2020;36:101671.
Kim J, Coetzee GA. Prostate specific antigen gene regulation by androgen receptor. J Cell Biochem. 2004;93:233–41.
Tkac J, Gajdosova V, Hroncekova S, Bertok T, Hires M, Jane E, et al. Prostate-specific antigen glycoprofiling as diagnostic and prognostic biomarker of prostate cancer. Interface Focus. 2019;9:20180077.
Solit DB, Scher HI, Rosen N. Hsp90 as a therapeutic target in prostate cancer. Semin Oncol. 2003;30:709–16.
Chen L, Li J, Farah E, Sarkar S, Ahmad N, Gupta S, et al. Cotargeting HSP90 and its client proteins for treatment of prostate cancer. Mol Cancer Ther. 2016;15:2107–18.
Moses MA, Kim YS, Rivera-Marquez GM, Oshima N, Watson MJ, Beebe KE, et al. Targeting the Hsp40/Hsp70 chaperone axis as a novel strategy to treat castration-resistant prostate cancer. Cancer Res. 2018;78:4022–35.
Funding
This study was funded by the National Natural Science Foundation of China (NSFC 81572542, NSFC 81874096), Research Project of Key Discipline of Guangdong Province (2019GDXK0010), Science and Technology Program of Guangzhou City (202102021130), National Key Specialty Construction Project of Clinical Pharmacy, High Level Clinical Key Specialty of Clinical Pharmacy in Guangdong Province, and Key Engineering Team, Discipline Cultvation Projects, Guangdong Pharmaceutical University (2024ZZ10).
Author information
Authors and Affiliations
Contributions
YLL:data collection and analysis, project development. DY:data collection and project development. WY, LSW, YYB, FYM, PYC, TRZ, FL, SHR, QXL, YBW, GYX, LYH: data collection and analysis. YLL and LX: manuscript writing. LX: idea development, data management and analysis and manuscript writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yin, L., Dai, Y., Wang, Y. et al. A mitochondrial outer membrane protein TOMM20 maintains protein stability of androgen receptor and regulates AR transcriptional activity in prostate cancer cells. Oncogene 44, 1567–1577 (2025). https://doi.org/10.1038/s41388-025-03328-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41388-025-03328-w
This article is cited by
-
Targeting Hsp70 triggers ferroptosis: a novel anti-cancer mechanism of a marine natural product in prostate cancer
Natural Products and Bioprospecting (2026)