Abstract
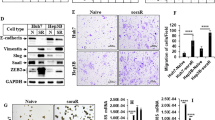
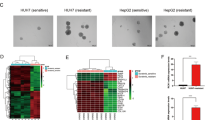
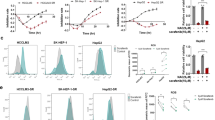
Sorafenib, a first-line targeted drug for advanced hepatocellular carcinoma (HCC), has limited clinical application due to intrinsic/acquired resistance. In this study, we have identified the RNA-binding protein RBMS3 as a pivotal regulator involved in sorafenib resistance among patients with HCC. Loss- and gain-of-function experiments further demonstrate that downregulation of RBMS3 promotes angiogenesis and confers resistance to sorafenib by augmenting the capacity of HCC cells to express and secrete ANGPT2, while upregulation of RBMS3 reverse these phenotypes.Through immunoprecipitation mass spectrometry experiments and co-immunoprecipitation (co-IP), we further verified that RBMS3 can facilitate the K48-linked ubiquitination and subsequent protein degradation of ANGPT2 by recruiting the ubiquitin E3 ligase TRIM21 in an RNA-independent manner.Additionally, RBMS3 is found to be deleted in HCC tissues and exhibits a significant positive correlation with angiogenesis and resistance to sorafenib treatment. Importantly, the combination of ANGPT2 antibody in RBMS3-deficient HCC cells restores sensitivity to sorafenib both in vitro and in vivo. These findings uncovered a novel molecular basis for post-translational upregulation of ANGPT2, suggesting that RBMS3-loss plays an oncogenic role in HCC by promoting angiogenesis and conferring resistance to sorafenib treatment.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49.
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clinicians. 2005;55:74–108.
Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155–66.
Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450–62.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90.
Keating GM, Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69:223–40.
Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–44.
Palmer DH. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:2498.
Tang W, Chen Z, Zhang W, Cheng Y, Zhang B, Wu F, et al. The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal Transduct Target Ther. 2020;5:87.
Sia D, Alsinet C, Newell P, Villanueva A. VEGF signaling in cancer treatment. Curr Pharm Des. 2014;20:2834–42.
Scartozzi M, Faloppi L, Svegliati Baroni G, Loretelli C, Piscaglia F, Iavarone M, et al. VEGF and VEGFR genotyping in the prediction of clinical outcome for HCC patients receiving sorafenib: the ALICE-1 study. Int J Cancer. 2014;135:1247–56.
Luo X, Feng GS. VEGFA genomic amplification tailors treatment of HCCs with sorafenib. Cancer Discov. 2014;4:640–1.
Moreno Garcia V, Basu B, Molife LR, Kaye SB. Combining antiangiogenics to overcome resistance: rationale and clinical experience. Clin Cancer Res. 2012;18:3750–61.
Morse MA, Sun W, Kim R, He AR, Abada PB, Mynderse M, et al. The Role of Angiogenesis in Hepatocellular Carcinoma. Clin Cancer Res. 2019;25:912–20.
Goh PP, Sze DM, Roufogalis BD. Molecular and cellular regulators of cancer angiogenesis. Curr Cancer Drug Targets. 2007;7:743–58.
Huang H, Lai JY, Do J, Liu D, Li L, Del Rosario J, et al. Specifically targeting angiopoietin-2 inhibits angiogenesis, Tie2-expressing monocyte infiltration, and tumor growth. Clin Cancer Res. 2011;17:1001–11.
Huang H, Bhat A, Woodnutt G, Lappe R. Targeting the ANGPT-TIE2 pathway in malignancy. Nat Rev Cancer. 2010;10:575–85.
Al-Moundhri MS, Al-Shukaili A, Al-Nabhani M, Al-Bahrani B, Burney IA, Rizivi A, et al. Measurement of circulating levels of VEGF-A, -C, and -D and their receptors, VEGFR-1 and -2 in gastric adenocarcinoma. World J Gastroenterol. 2008;14:3879–83.
Tsai JH, Lee WM. Tie2 in tumor endothelial signaling and survival: implications for antiangiogenic therapy. Mol Cancer Res. 2009;7:300–10.
Glisovic T, Bachorik JL, Yong J, Dreyfuss G. RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett. 2008;582:1977–86.
Kashikar R, Kotha AK, Shah S, Famta P, Singh SB, Srivastava S, et al. Advances in nanoparticle mediated targeting of RNA binding protein for cancer. Adv Drug Deliv Rev. 2022;185:114257.
Wu X, Xu L. The RNA-binding protein HuR in human cancer: A friend or foe? Adv Drug Deliv Rev. 2022;184:114179.
Keene JD. RNA regulons: coordination of post-transcriptional events. Nat Rev Genet. 2007;8:533–43.
Penkov D, Ni R, Else C, Pinol-Roma S, Ramirez F, Tanaka S. Cloning of a human gene closely related to the genes coding for the c-myc single-strand binding proteins. Gene. 2000;243:27–36.
Ruan X, Liu Y, Wang P, Liu L, Ma T, Xue Y, et al. RBMS3-induced circHECTD1 encoded a novel protein to suppress the vasculogenic mimicry formation in glioblastoma multiforme. Cell Death Dis. 2023;14:745.
Vaishnavi A, Juan J, Jacob M, Stehn C, Gardner EE, Scherzer MT, et al. Transposon Mutagenesis Reveals RBMS3 Silencing as a Promoter of Malignant Progression of BRAFV600E-Driven Lung Tumorigenesis. Cancer Res. 2022;82:4261–73.
Gornicki T, Lambrinow J, Mrozowska M, Podhorska-Okolow M, Dziegiel P, Grzegrzolka J. Role of RBMS3 Novel Potential Regulator of the EMT Phenomenon in Physiological and Pathological Processes. Int J Mol Sci. 2022;23:10875.
Zhu L, Xi PW, Li XX, Sun X, Zhou WB, Xia TS, et al. The RNA binding protein RBMS3 inhibits the metastasis of breast cancer by regulating Twist1 expression. J Exp Clin Cancer Res. 2019;38:105.
Li Y, Chen L, Nie CJ, Zeng TT, Liu H, Mao X, et al. Downregulation of RBMS3 is associated with poor prognosis in esophageal squamous cell carcinoma. Cancer Res. 2011;71:6106–15.
Wu G, Cao L, Zhu J, Tan Z, Tang M, Li Z, et al. Loss of RBMS3 Confers Platinum Resistance in Epithelial Ovarian Cancer via Activation of miR-126-5p/beta-catenin/CBP signaling. Clin Cancer Res. 2019;25:1022–35.
Gyorffy B. Integrated analysis of public datasets for the discovery and validation of survival-associated genes in solid tumors. Innovation. 2024;5:100625.
Deng F, Zhou R, Lin C, Yang S, Wang H, Li W, et al. Tumor-secreted dickkopf2 accelerates aerobic glycolysis and promotes angiogenesis in colorectal cancer. Theranostics. 2019;9:1001–14.
Guelfi S, Hodivala-Dilke K, Bergers G. Targeting the tumour vasculature: from vessel destruction to promotion. Nat Rev Cancer. 2024;24:655–75.
Ladd AD, Duarte S, Sahin I, Zarrinpar A. Mechanisms of drug resistance in HCC. Hepatology. 2024;79:926–40.
Hu C, Li W, Tian F, Jiang K, Liu X, Cen J, et al. Arid1a regulates response to anti-angiogenic therapy in advanced hepatocellular carcinoma. J Hepatol. 2018;68:465–75.
Zhu J, Wu Y, Yu Y, Li Y, Shen J, Zhang R. MYBL1 induces transcriptional activation of ANGPT2 to promote tumor angiogenesis and confer sorafenib resistance in human hepatocellular carcinoma. Cell Death Dis. 2022;13:727.
Urosevic J, Blasco MT, Llorente A, Bellmunt A, Berenguer-Llergo A, Guiu M, et al. ERK1/2 Signaling Induces Upregulation of ANGPT2 and CXCR4 to Mediate Liver Metastasis in Colon Cancer. Cancer Res. 2020;80:4668–80.
Park HR, Shiva A, Cummings P, Kim S, Kim S, Lee E, et al. Angiopoietin-2-Dependent Spatial Vascular Destabilization Promotes T-cell Exclusion and Limits Immunotherapy in Melanoma. Cancer Res. 2023;83:1968–83.
Alomari M. TRIM21 - A potential novel therapeutic target in cancer. Pharm Res. 2021;165:105443.
Chen X, Cao M, Wang P, Chu S, Li M, Hou P, et al. The emerging roles of TRIM21 in coordinating cancer metabolism, immunity and cancer treatment. Front Immunol. 2022;13:968755.
Liu YX, Wan S, Yang XQ, Wang Y, Gan WJ, Ye WL, et al. TRIM21 is a druggable target for the treatment of metastatic colorectal cancer through ubiquitination and activation of MST2. Cell Chem Biol. 2023;30:709–25.e6.
Wan S, He QY, Yang Y, Liu F, Zhang X, Guo X, et al. SPARC Stabilizes ApoE to Induce Cholesterol-Dependent Invasion and Sorafenib Resistance in Hepatocellular Carcinoma. Cancer Res. 2024;84:1872–88.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No.82103637, 82303401), Guangzhou Municipal Science and Technology Project (2024A04J3475, 2025A03J3756), Joint provincial and municipal fund in Guangdong Province (2022A1515111204) and Discipline Excellence Program of Guangdong Pharmaceutical University (2024QZ06).
Author information
Authors and Affiliations
Contributions
J. Zhu, G. Wu and S. Zhang supervise the project, review and editing the manuscript. S. Ou and X. Nie performed all the in vitro experiments. L. Wang conducted the molecular cloning. J. Zhu and L. Wang performed IHC assay. J. Shen provided patient tissue samples and analyzed clinical data. J. Zhu and X. Nie performed in vivo experiments. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, J., Wang, L., Nie, X. et al. RBMS3-loss impedes TRIM21-induced ubiquitination of ANGPT2 in an RNA-independent manner and drives sorafenib resistance in hepatocellular carcinoma. Oncogene 44, 1620–1633 (2025). https://doi.org/10.1038/s41388-025-03335-x
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41388-025-03335-x