Abstract
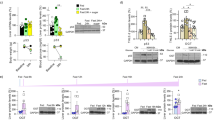
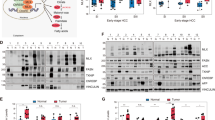
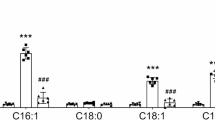
Excessive glycogen deposition is a common feature of liver enlargement, liver adenoma, and liver cancer, yet the underlying mechanisms remain poorly understood. In this study, we found that NUMB, a well-known cell fate determinant, is downregulated in glycogen-rich adenomas and hepatocellular carcinoma (HCC). NUMB-deficient livers developed excessive glycogen accumulation and adenoma formation particularly in aged mice. Surprisingly, the Alb-Cre:Trp53loxP/loxP liver displayed no similar defective morphology and function, although p53 is considered an important downstream target of NUMB and closely related to glucose metabolism. Instead, we observed a synergistic interaction between NUMB and p53 in regulating glycogen metabolism in HCC tissues and cell lines. Combined knockout of NUMB and p53 in mice significantly enhances glycogen accumulation and hepatomegaly, particularly when mice are subjected to a high sugar diet (HSD), leading to higher cancer incidence. Mechanistically, NUMB deficiency disrupts the PTEN-PI3K/AKT signaling pathway, promoting glycogen accumulation. Subsequently, successive glycogen deposition triggers hepatomegaly and tumorigenesis via the Hippo signaling pathway. Our results suggest that NUMB plays a crucial role in maintaining the homeostasis of glucose metabolism and suppressing the development of liver tumors associated with glycogen deposition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Kwok MK, Lewin KJ. Massive hepatomegaly in adult polycystic liver disease. Am J Surg Pathol. 1988;12:321–4.
Ebert EC, Nagar M, Hagspiel KD. Gastrointestinal and hepatic complications of sickle cell disease. Clin Gastroenterol Hepatol. 2010;8:483–9. quiz.e70.
He F, Antonucci L, Yamachika S, Zhang Z, Taniguchi K, Umemura A, et al. NRF2 activates growth factor genes and downstream AKT signaling to induce mouse and human hepatomegaly. J Hepatol. 2020;72:1182–95.
Bianchi L. Glycogen storage disease I and hepatocellular tumours. Eur J Pediatr. 1993;152:S63–70.
Chatila R, West AB. Hepatomegaly and abnormal liver tests due to glycogenosis in adults with diabetes. Medicine. 1996;75:327–33.
Wilson LH, Cho JH, Estrella A, Smyth JA, Wu R, Chengsupanimit T, et al. Liver Glycogen Phosphorylase Deficiency Leads to Profibrogenic Phenotype in a Murine Model of Glycogen Storage Disease Type VI. Hepatol Commun. 2019;3:1544–55.
Liu Q, Li J, Zhang W, Xiao C, Zhang S, Nian C, et al. Glycogen accumulation and phase separation drives liver tumor initiation. Cell. 2021;184:5559–76.e19.
Yu FX, Zhao B, Guan KL. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell. 2015;163:811–28.
Cox AG, Hwang KL, Brown KK, Evason K, Beltz S, Tsomides A, et al. Yap reprograms glutamine metabolism to increase nucleotide biosynthesis and enable liver growth. Nat Cell Biol. 2016;18:886–96.
Zhang S, Zhou D. Role of the transcriptional coactivators YAP/TAZ in liver cancer. Curr Opin Cell Biol. 2019;61:64–71.
Hay N. Interplay between FOXO, TOR, and Akt. Biochim Biophys Acta. 2011;1813:1965–70.
Ono H, Shimano H, Katagiri H, Yahagi N, Sakoda H, Onishi Y, et al. Hepatic Akt activation induces marked hypoglycemia, hepatomegaly, and hypertriglyceridemia with sterol regulatory element binding protein involvement. Diabetes. 2003;52:2905–13.
Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets. 2008;8:187–98.
Shao C, Li Z, Ahmad N, Liu X. Regulation of PTEN degradation and NEDD4-1 E3 ligase activity by Numb. Cell Cycle. 2017;16:957–67.
Ye L, Lou F, Yu F, Zhang D, Wang C, Wu F, et al. NUMB maintains bone mass by promoting degradation of PTEN and GLI1 via ubiquitination in osteoblasts. Bone Res. 2018;6:32.
Shu Y, Xu Q, Xu Y, Tao Q, Shao M, Cao X, et al. Loss of Numb promotes hepatic progenitor expansion and intrahepatic cholangiocarcinoma by enhancing Notch signaling. Cell Death Dis. 2021;12:966.
Colaluca IN, Tosoni D, Nuciforo P, Senic-Matuglia F, Galimberti V, Viale G, et al. NUMB controls p53 tumour suppressor activity. Nature. 2008;451:76–80.
Jiang P, Du W, Wang X, Mancuso A, Gao X, Wu M, et al. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat Cell Biol. 2011;13:310–6.
Chen SL, Zhang CZ, Liu LL, Lu SX, Pan YH, Wang CH, et al. A GYS2/p53 Negative Feedback Loop Restricts Tumor Growth in HBV-Related Hepatocellular Carcinoma. Cancer Res. 2019;79:534–45.
Wu X, Bayle JH, Olson D, Levine AJ. The p53-mdm-2 autoregulatory feedback loop. Genes Dev. 1993;7:1126–32.
Fan X, Wang R, Song Y, Wang Z, Wang X, Liu Y, et al. Effects of high-sugar, high-cholesterol, and high-fat diet on phospholipid profile of mouse tissues with a focus on the mechanism of plasmalogen synthesis. Biochim Biophys Acta Mol Cell Biol Lipids. 2023;1868:159345.
Su D, Li Y, Zhang W, Gao H, Cheng Y, Hou Y, et al. SPTAN1/NUMB axis senses cell density to restrain cell growth and oncogenesis through Hippo signaling. J Clin Invest. 2023;133:e168888.
Sczaniecka M, Gladstone K, Pettersson S, McLaren L, Huart AS, Wallace M. MDM2 protein-mediated ubiquitination of numb protein: identification of a second physiological substrate of MDM2 that employs a dual-site docking mechanism. J Biol Chem. 2012;287:14052–68.
Liu XH, Yao S, Levine AC, Kirschenbaum A, Pan J, Wu Y, et al. Nandrolone, an anabolic steroid, stabilizes Numb protein through inhibition of mdm2 in C2C12 myoblasts. J Androl. 2012;33:1216–23.
Uko NE, Guner OF, Matesic DF, Bowen JP. Akt Pathway Inhibitors. Curr Top Med Chem. 2020;20:883–900.
Manmadhan S, Ehmer U. Hippo Signaling in the Liver - A Long and Ever-Expanding Story. Front Cell Dev Biol. 2019;7:33.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Curtis M, Kenny HA, Ashcroft B, Mukherjee A, Johnson A, Zhang Y, et al. Fibroblasts Mobilize Tumor Cell Glycogen to Promote Proliferation and Metastasis. Cell Metab. 2019;29:141–55.e9.
Favaro E, Bensaad K, Chong MG, Tennant DA, Ferguson DJ, Snell C, et al. Glucose utilization via glycogen phosphorylase sustains proliferation and prevents premature senescence in cancer cells. Cell Metab. 2012;16:751–64.
McBride A, Ghilagaber S, Nikolaev A, Hardie DG. The glycogen-binding domain on the AMPK beta subunit allows the kinase to act as a glycogen sensor. Cell Metab. 2009;9:23–34.
Chou JY, Jun HS, Mansfield BC. Glycogen storage disease type I and G6Pase-beta deficiency: etiology and therapy. Nat Rev Endocrinol. 2010;6:676–88.
Franco LM, Krishnamurthy V, Bali D, Weinstein DA, Arn P, Clary B, et al. Hepatocellular carcinoma in glycogen storage disease type Ia: a case series. J Inherit Metab Dis. 2005;28:153–62.
Tucci FA, Pennisi R, Rigiracciolo DC, Filippone MG, Bonfanti R, Romeo F, et al. Loss of NUMB drives aggressive bladder cancer via a RHOA/ROCK/YAP signaling axis. Nat Commun. 2024;15:10378.
Lv Z, Ren Y, Li Y, Niu F, Li Z, Li M, et al. RNA-binding protein GIGYF2 orchestrates hepatic insulin resistance through STAU1/PTEN-mediated disruption of the PI3K/AKT signaling cascade. Mol Med. 2024;30:124.
El-Zeftawy M, Ghareeb D, ElBealy ER, Saad R, Mahmoud S, Elguindy N, et al. Berberine chloride ameliorated PI3K/Akt-p/SIRT-1/PTEN signaling pathway in insulin resistance syndrome induced in rats. J Food Biochem. 2019;43:e13049.
Chen G, Fan XY, Zheng XP, Jin YL, Liu Y, Liu SC. Human umbilical cord-derived mesenchymal stem cells ameliorate insulin resistance via PTEN-mediated crosstalk between the PI3K/Akt and Erk/MAPKs signaling pathways in the skeletal muscles of db/db mice. Stem Cell Res Ther. 2020;11:401.
Trotman LC, Pandolfi PP. PTEN and p53: who will get the upper hand?. Cancer Cell. 2003;3:97–9.
Nakanishi A, Kitagishi Y, Ogura Y, Matsuda S. The tumor suppressor PTEN interacts with p53 in hereditary cancer (Review). Int J Oncol. 2014;44:1813–9.
Munoz-Fontela C, Mandinova A, Aaronson SA, Lee SW. Emerging roles of p53 and other tumour-suppressor genes in immune regulation. Nat Rev Immunol. 2016;16:741–50.
Acknowledgements
We thank Guang Yang and Guangneng Liao for their help with mouse management and breeding.
Funding
This study was supported by grants from the Natural Science Foundation of China (U22A20343, 82200691, and 82404060); the Key Research and Development Program and the Young Scientists Fund of the Natural Science Foundation of Sichuan Province (2023YFS0041, 2024NSFSC1901, and 2024NSFSC0637); the Postdoctor Research Fund of West China Hospital, Sichuan University (2024HXBH083).
Author information
Authors and Affiliations
Contributions
Yuke Shu and Qing Tao performed most of the experiments equally and wrote this manuscript; Yujun Shi, Chuan Li and Qing Xu designed and supervised the study and revised this manuscript; Zhenru Wu, Yahong Xu and Menglin Chen performed histology examination; Tingting Ma, Yuwei Chen, Fei Liu and Zhiqi Zhu collected clinical data; Xinyu Wei, Mingyang Shao,Xiaoyue Cao, Yuwei Chen, and Yuting Zeng performed informatic analysis; Yongjie Zhou and Wei Peng gave critical suggestions. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The animal care and experimental procedures were conducted in accordance with national and international laws and policies and were approved by the Animal Care and Use Committee of Sichuan University(Approval No. 20230206006). All patient materials were obtained with written informed consent. Approval for this study was granted by the Ethics Committee of the West China Hospital of Sichuan University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shu, Y., Tao, Q., Xu, Q. et al. Loss of NUMB promotes hepatomegaly and hepatocellular carcinoma through the AKT/glycogen/hippo signaling. Oncogene 44, 2574–2587 (2025). https://doi.org/10.1038/s41388-025-03430-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41388-025-03430-z