Abstract
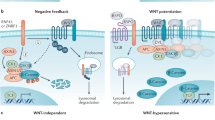
Tumor suppressor genes (TSGs) that regulate the stemness of lung cancer cells remain to be determined. We conducted a genome-wide CRISPR/Cas9-mediated screening and identified REPS2 as a potent TSG that negatively regulates the stemness of lung cancer cells. Its tumor suppressive function was confirmed both in vitro and in vivo. Mechanistically, P62 interacts simultaneously with both β-catenin and REPS2, leading to autophagy-lysosome-mediated degradation of β-catenin and attenuation of Wnt signaling. A β-catenin inhibitor synergizes with inhibitors for driver mutants to induce immunogenic cell death, which could be exploited for enhancing efficacy of tumor immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All the data obtained and/or analyzed during the current study were available from the corresponding authors on reasonable request.
References
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63.
Zheng M. Classification and pathology of lung cancer. Surg Oncol Clin N Am. 2016;25:447–68.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30.
Lai D, Visser-Grieve S, Yang X. Tumour suppressor genes in chemotherapeutic drug response. Biosci Rep. 2012;32:361–74.
Yeddula N, Xia Y, Ke E, Beumer J, Verma IM. Screening for tumor suppressors: loss of ephrin receptor A2 cooperates with oncogenic KRas in promoting lung adenocarcinoma. Proc Natl Acad Sci USA. 2015;112:E6476–85.
Gao L, Hu Y, Tian Y, Fan Z, Wang K, Li H, et al. Lung cancer deficient in the tumor suppressor GATA4 is sensitive to TGFBR1 inhibition. Nat Commun. 2019;10:1665.
Chen Z, Fan Z, Dou X, Zhou Q, Zeng G, Liu L, et al. Inactivation of tumor suppressor gene Clusterin leads to hyperactivation of TAK1-NF-kappaB signaling axis in lung cancer cells and denotes a therapeutic opportunity. Theranostics. 2020;10:11520–34.
Zhou Q, Chen W, Fan Z, Chen Z, Liang J, Zeng G, et al. Targeting hyperactive TGFBR2 for treating MYOCD deficient lung cancer. Theranostics. 2021;11:6592–606.
Liu L, Lei Y, Chen W, Zhou Q, Zheng Z, Zeng G, et al. In vivo genome-wide CRISPR screening identifies ZNF24 as a negative NF-kappaB modulator in lung cancer. Cell Biosci. 2022;12:193.
Bao B, Ahmad A, Azmi AS, Ali S, Sarkar FH. Overview of cancer stem cells (CSCs) and mechanisms of their regulation: implications for cancer therapy. Curr Protoc Pharmacol. 2013;Chapter 14:Unit 14.25.
Zhou HM, Zhang JG, Zhang X, Li Q. Targeting cancer stem cells for reversing therapy resistance: mechanism, signaling, and prospective agents. Signal Transduct Target Ther. 2021;6:62.
Kilmister EJ, Koh SP, Weth FR, Gray C, Tan ST. Cancer metastasis and treatment resistance: mechanistic insights and therapeutic targeting of cancer stem cells and the tumor microenvironment. Biomedicines. 2022;10:2988.
Chang JC. Cancer stem cells: role in tumor growth, recurrence, metastasis, and treatment resistance. Medicine. 2016;95:S20–5.
Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5:8.
Zeng Z, Fu M, Hu Y, Wei Y, Wei X, Luo M. Regulation and signaling pathways in cancer stem cells: implications for targeted therapy for cancer. Mol Cancer. 2023;22:172.
Ikeda M, Ishida O, Hinoi T, Kishida S, Kikuchi A. Identification and characterization of a novel protein interacting with Ral-binding protein 1, a putative effector protein of Ral. J Biol Chem. 1998;273:814–21.
Polo S, Confalonieri S, Salcini AE, Di Fiore PP. EH and UIM: endocytosis and more. Sci STKE. 2003;2003:re17.
Zhang H, Duan CJ, Zhang H, Cheng YD, Zhang CF. Expression and clinical significance of REPS2 in human esophageal squamous cell carcinoma. Asian Pac J Cancer Prev. 2013;14:2851–7.
Penninkhof F, Grootegoed JA, Blok LJ. Identification of REPS2 as a putative modulator of NF-kappaB activity in prostate cancer cells. Oncogene. 2004;23:5607–15.
He XY, Zhu MM, Zheng J, Wang CY, Zhao XK, Zhang BT, et al. Liver X receptor agonists exert antitumor effects against hepatocellular carcinoma via inducing REPS2 expression. Acta Pharmacol Sin. 2023;44:635–46.
Sethi JK, Vidal-Puig A. Wnt signalling and the control of cellular metabolism. Biochem J. 2010;427:1–17.
Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, et al. Wnt/beta-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7:3.
Valkenburg KC, Graveel CR, Zylstra-Diegel CR, Zhong Z, Williams BO. Wnt/beta-catenin signaling in normal and cancer stem cells. Cancers. 2011;3:2050–79.
Ye L, Xiang T, Fan Y, Zhang D, Li L, Zhang C, et al. The 19q13 KRAB Zinc-finger protein ZFP82 suppresses the growth and invasion of esophageal carcinoma cells through inhibiting NF-kappaB transcription and inducing apoptosis. Epigenomics. 2019;11:65–80.
Hua C, Chen J, Li S, Zhou J, Fu J, Sun W, et al. KDM6 demethylases and their roles in human cancers. Front Oncol. 2021;11:779918.
Chen X, Zhang H, Aravindakshan JP, Gotlieb WH, Sairam MR. Anti-proliferative and pro-apoptotic actions of a novel human and mouse ovarian tumor-associated gene OTAG-12: downregulation, alternative splicing and drug sensitization. Oncogene. 2011;30:2874–87.
Jaggupilli A, Elkord E. Significance of CD44 and CD24 as cancer stem cell markers: an enduring ambiguity. Clin Dev Immunol. 2012;2012:708036.
Komuro H, Saihara R, Shinya M, Takita J, Kaneko S, Kaneko M, et al. Identification of side population cells (stem-like cell population) in pediatric solid tumor cell lines. J Pediatr Surg. 2007;42:2040–5.
Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, et al. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA. 2004;101:14228–33.
Ribatti D, Tamma R, Annese T. Epithelial-mesenchymal transition in cancer: a historical overview. Transl Oncol. 2020;13:100773.
Zhang Y, Zhang X, Huang X, Tang X, Zhang M, Li Z, et al. Tumor stemness score to estimate epithelial-to-mesenchymal transition (EMT) and cancer stem cells (CSCs) characterization and to predict the prognosis and immunotherapy response in bladder urothelial carcinoma. Stem Cell Res Ther. 2023;14:15.
Haslehurst AM, Koti M, Dharsee M, Nuin P, Evans K, Geraci J, et al. EMT transcription factors snail and slug directly contribute to cisplatin resistance in ovarian cancer. BMC Cancer. 2012;12:91.
Li F, Song X, Li X, Zhang X, Feng X, Wang L, et al. Lgr5 maintains stemness and regulates cell property in nasopharyngeal carcinoma through Wnt/beta-catenin signaling pathway. Stem Cell Res. 2020;47:101916.
Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, et al. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected]. Proc Natl Acad Sci USA. 2004;101:12682–7.
Szewczyk LM, Lipiec MA, Liszewska E, Meyza K, Urban-Ciecko J, Kondrakiewicz L, et al. Astrocytic beta-catenin signaling via TCF7L2 regulates synapse development and social behavior. Mol Psychiatry. 2024;29:57–73.
Bustamante HA, Gonzalez AE, Cerda-Troncoso C, Shaughnessy R, Otth C, Soza A, et al. Interplay between the autophagy-lysosomal pathway and the ubiquitin-proteasome system: a target for therapeutic development in Alzheimer’s disease. Front Cell Neurosci. 2018;12:126.
Hale AN, Ledbetter DJ, Gawriluk TR, Rucker EB, 3rd. Autophagy: regulation and role in development. Autophagy. 2013;9:951–72.
Tanida I, Ueno T, Kominami E. LC3 and autophagy. Methods Mol Biol. 2008;445:77–88.
Runwal G, Stamatakou E, Siddiqi FH, Puri C, Zhu Y, Rubinsztein DC. LC3-positive structures are prominent in autophagy-deficient cells. Sci Rep. 2019;9:10147.
Kumar AV, Mills J, Lapierre LR. Selective autophagy receptor p62/SQSTM1, a pivotal player in stress and aging. Front Cell Dev Biol. 2022;10:793328.
Chen H, Zou Y, Shang Y, Lin H, Wang Y, Cai R, et al. Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physiol. 2008;146:368–76.
Mustachio LM, Chelariu-Raicu A, Szekvolgyi L, Roszik J. Targeting KRAS in cancer: promising therapeutic strategies. Cancers. 2021;13:1204.
Ji H, Wang Z, Perera SA, Li D, Liang MC, Zaghlul S, et al. Mutations in BRAF and KRAS converge on activation of the mitogen-activated protein kinase pathway in lung cancer mouse models. Cancer Res. 2007;67:4933–9.
Wu PK, Park JI. MEK1/2 inhibitors: molecular activity and resistance mechanisms. Semin Oncol. 2015;42:849–62.
Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72.
Rouzbahani E, Majidpoor J, Najafi S, Mortezaee K. Cancer stem cells in immunoregulation and bypassing anti-checkpoint therapy. Biomed Pharmacother. 2022;156:113906.
Ashrafi A, Akter Z, Modareszadeh P, Modareszadeh P, Berisha E, Alemi PS, et al. Current Landscape of therapeutic resistance in lung cancer and promising strategies to overcome resistance. Cancers. 2022;14:4562.
Barbato L, Bocchetti M, Di Biase A, Regad T. Cancer stem cells and targeting strategies. Cells. 2019;8:926.
Manni W, Min W. Signaling pathways in the regulation of cancer stem cells and associated targeted therapy. MedComm. 2022;3:e176.
Karami Fath M, Ebrahimi M, Nourbakhsh E, Zia Hazara A, Mirzaei A, Shafieyari S, et al. PI3K/Akt/mTOR signaling pathway in cancer stem cells. Pathol Res Pract. 2022;237:154010.
Qian L, Mahaffey JP, Alcorn HL, Anderson KV. Tissue-specific roles of Axin2 in the inhibition and activation of Wnt signaling in the mouse embryo. Proc Natl Acad Sci USA. 2011;108:8692–7.
Schneikert J, Behrens J. The canonical Wnt signalling pathway and its APC partner in colon cancer development. Gut. 2007;56:417–25.
Groenewald W, Lund AH, Gay DM. The role of WNT pathway mutations in cancer development and an overview of therapeutic options. Cells. 2023;12:990.
Del Valle-Perez B, Arques O, Vinyoles M, de Herreros AG, Dunach M. Coordinated action of CK1 isoforms in canonical Wnt signaling. Mol Cell Biol. 2011;31:2877–88.
Adriaenssens E, Ferrari L, Martens S. Orchestration of selective autophagy by cargo receptors. Curr Biol. 2022;32:R1357–71.
Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86.
Abi-Aad SJ, Zouein J, Chartouni A, Naim N, Kourie HR. Simultaneous inhibition of PD-1 and LAG-3: the future of immunotherapy? Immunotherapy. 2023;15:611–8.
Chu X, Tian W, Wang Z, Zhang J, Zhou R. Co-inhibition of TIGIT and PD-1/PD-L1 in cancer immunotherapy: mechanisms and clinical trials. Mol Cancer. 2023;22:93.
Sordo-Bahamonde C, Lorenzo-Herrero S, Gonzalez-Rodriguez AP, Martinez-Perez A, Rodrigo JP, Garcia-Pedrero JM, et al. Chemo-immunotherapy: a new trend in cancer treatment. Cancers. 2023;15:2912.
Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–51.
Yardley DA. Drug resistance and the role of combination chemotherapy in improving patient outcomes. Int J Breast Cancer. 2013;2013:137414.
Funding
This work was financially supported by Key Technologies R&D Program of Guangdong Province (2023B1111030003) and the National Key Research and Development Program of China (No. 2022YFA1103900) to LC, Guangdong Basic and Applied Basic Research Foundation (2024A1515030238), and Guangzhou Science and Technology program City-University Joint Funding Project (2024A03J0596) to QZ.
Author information
Authors and Affiliations
Contributions
LL, SC, and XY performed most of the experiments, analyzed the data, and contributed to the manuscript composition. LL, SC, and XY performed in vitro cell line studies, western blot, real-time PCR, and the public dataset analysis. ZL, RZ, GZ, and ZZ performed animal experiments. QZ and WL performed screening through CRISPR/Cas9 and did bioinformatics and significance calculation. LC and QZ designed experiments, analyzed results, supervised the project, and wrote the manuscript. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All the animal experimental protocols were approved by the Committee of the Institute of Animal Protection and the use of Jinan University. All animal experiments were carried out in accordance with the approved protocols.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g., a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, L., Chen, S., Lei, Y. et al. REPS2 attenuates cancer stemness through inhibiting Wnt signaling by autophagy mediated degradation of β-catenin. Oncogene 44, 2942–2955 (2025). https://doi.org/10.1038/s41388-025-03469-y
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41388-025-03469-y