Abstract
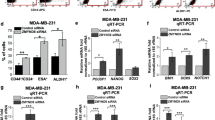
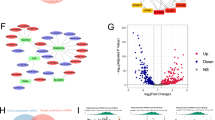
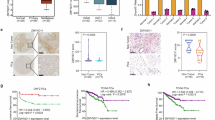
Pancreatic cancer (PC) is a digestive tract tumour with an extremely poor patient prognosis and prominent metabolic abnormalities. However, the molecular mechanisms underlying metabolic reprogramming in the progression of pancreatic cancer remain poorly understood. Here, we employed an epigenetic siRNA library to identify a crucial regulator, ZMYND8, which is involved in glycolysis in PC cells. ZMYND8 was frequently overexpressed in both PC tissues and cell lines, and its elevated expression was significantly correlated with poor overall survival in patients with PC. The high rates of glucose uptake and lactate secretion conferred by ZMYND8 revealed an abnormal activity of aerobic glycolysis in PC cells. Functional studies revealed that ZMYND8 significantly promoted the proliferation, migration and invasion of PC cells. Integrated analyses of CUT&Tag and RNA-seq data revealed that ZMYND8 may activate c-Myc transcriptional activity by modulating downstream epigenetic regulatory pathways. Proteomic profiling and coimmunoprecipitation (Co-IP) assays further demonstrated a direct physical interaction between ZMYND8 and c-Myc. Mechanistic studies revealed that ZMYND8 interacted with and activated c-Myc, thereby promoting the Warburg effect and facilitating PC cell malignancy. Moreover, in vivo studies revealed that overexpression of ZMYND8 resulted in accelerated tumour growth in PC xenografts, which was reversible through the knockdown of c-Myc or treatment with 2-deoxy-D-glucose. Collectively, our data suggest that ZMYND8 functions as a critical metabolic regulator in PC cells by tightly regulating c-Myc activity and may represent a promising novel therapeutic target for advanced pancreatic cancer treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12–49.
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63.
George B. Precision medicine and pancreatic cancer. Surg Oncol Clin N Am. 2021;30:693–708.
Buscail L, Bournet B, Cordelier P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2020;17:153–68.
Chen M, Cen K, Song Y, Zhang X, Liou YC, Liu P, et al. NUSAP1-LDHA-Glycolysis-Lactate feedforward loop promotes Warburg effect and metastasis in pancreatic ductal adenocarcinoma. Cancer Lett. 2023;567:216285.
Kant R, Manne RK, Anas M, Penugurti V, Chen T, Pan BS, et al. Deregulated transcription factors in cancer cell metabolisms and reprogramming. Semin Cancer Biol. 2022;86:1158–74.
Yang J, Ren B, Yang G, Wang H, Chen G, You L, et al. The enhancement of glycolysis regulates pancreatic cancer metastasis. Cell Mol Life Sci. 2020;77:305–21.
Vaupel P, Schmidberger H, Mayer A. The Warburg effect: essential part of metabolic reprogramming and central contributor to cancer progression. Int J Radiat Biol. 2019;95:912–9.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Sun K, Zhang X, Shi J, Huang J, Wang S, Li X, et al. Elevated protein lactylation promotes immunosuppressive microenvironment and therapeutic resistance in pancreatic ductal adenocarcinoma. J Clin Invest. 2025;135:e187024.
Ghosh K, Tang M, Kumari N, Nandy A, Basu S, Mall DP, et al. Positive regulation of transcription by human ZMYND8 through its association with P-TEFb complex. Cell Rep. 2018;24:2141–54 e2146.
Mukherjee S, Adhikary S, Gadad SS, Mondal P, Sen S, Choudhari R, et al. Suppression of poised oncogenes by ZMYND8 promotes chemo-sensitization. Cell Death Dis. 2020;11:1073.
Dou C, Mo H, Chen T, Liu J, Zeng Y, Li S, et al. ZMYND8 promotes the growth and metastasis of hepatocellular carcinoma by promoting HK2-mediated glycolysis. Pathol Res Pr. 2021;219:153345.
Luo M, Bao L, Chen Y, Xue Y, Wang Y, Zhang B, et al. ZMYND8 is a master regulator of 27-hydroxycholesterol that promotes tumorigenicity of breast cancer stem cells. Sci Adv. 2022;8:eabn5295.
Shen H, Xu W, Guo R, Rong B, Gu L, Wang Z, et al. Suppression of enhancer overactivation by a RACK7-histone demethylase complex. Cell. 2016;165:331–42.
Scognamiglio R, Cabezas-Wallscheid N, Thier MC, Altamura S, Reyes A, Prendergast AM, et al. Myc depletion induces a pluripotent dormant state mimicking diapause. Cell. 2016;164:668–80.
Farrell AS, Joly MM, Allen-Petersen BL, Worth PJ, Lanciault C, Sauer D, et al. MYC regulates ductal-neuroendocrine lineage plasticity in pancreatic ductal adenocarcinoma associated with poor outcome and chemoresistance. Nat Commun. 2017;8:1728.
Xia P, Zhang H, Lu H, Xu K, Jiang X, Jiang Y, et al. METTL5 stabilizes c-Myc by facilitating USP5 translation to reprogram glucose metabolism and promote hepatocellular carcinoma progression. Cancer Commun. 2023;43:338–64.
Zhang H, Zhai X, Liu Y, Xia Z, Xia T, Du G, et al. NOP2-mediated m5C modification of c-Myc in an EIF3A-dependent manner to reprogram glucose metabolism and promote hepatocellular carcinoma progression. Research. 2023;6:0184.
Wang H, Wang J, Liu J, Wang Y, Xia G, Huang X. Jumonji-C domain-containing protein 5 suppresses proliferation and aerobic glycolysis in pancreatic cancer cells in a c-Myc-dependent manner. Cell Signal. 2022;93:110282.
Cao Z, Budinich KA, Huang H, Ren D, Lu B, Zhang Z, et al. ZMYND8-regulated IRF8 transcription axis is an acute myeloid leukemia dependency. Mol Cell. 2021;81:3604–22 e3610.
Kosti A, de Araujo PR, Li WQ, Guardia GDA, Chiou J, Yi C, et al. The RNA-binding protein SERBP1 functions as a novel oncogenic factor in glioblastoma by bridging cancer metabolism and epigenetic regulation. Genome Biol. 2020;21:195.
Gunda V, Souchek J, Abrego J, Shukla SK, Goode GD, Vernucci E, et al. MUC1-mediated metabolic alterations regulate response to radiotherapy in pancreatic cancer. Clin Cancer Res. 2017;23:5881–91.
Hossain MA, Claggett JM, Edwards SR, Shi A, Pennebaker SL, Cheng MY, et al. Posttranscriptional regulation of Gcr1 expression and activity is crucial for metabolic adjustment in response to glucose availability. Mol Cell. 2016;62:346–58.
Tang B, Sun R, Wang D, Sheng H, Wei T, Wang L, et al. ZMYND8 preferentially binds phosphorylated EZH2 to promote a PRC2-dependent to -independent function switch in hypoxia-inducible factor-activated cancer. Proc Natl Acad Sci USA. 2021;118:e2019052118.
Fang Y, Tang W, Qu S, Li Z, Zhang X, Miao Y, et al. RBBP7, regulated by SP1, enhances the Warburg effect to facilitate the proliferation of hepatocellular carcinoma cells via PI3K/AKT signaling. J Transl Med. 2024;22:170.
Chen Y, Tsai YH, Tseng SH. Regulation of ZMYND8 to treat cancer. Molecules. 2021;26:1083.
Wang H, Sun J, Sun H, Wang Y, Lin B, Wu L, et al. The OGT-c-Myc-PDK2 axis rewires the TCA cycle and promotes colorectal tumor growth. Cell Death Differ. 2024;31:1157–69.
Spruijt CG, Luijsterburg MS, Menafra R, Lindeboom RG, Jansen PW, Edupuganti RR, et al. ZMYND8 co-localizes with NuRD on target genes and regulates poly(ADP-Ribose)-dependent recruitment of GATAD2A/NuRD to sites of DNA damage. Cell Rep. 2016;17:783–98.
Chen Y, Zhang B, Bao L, Jin L, Yang M, Peng Y, et al. ZMYND8 acetylation mediates HIF-dependent breast cancer progression and metastasis. J Clin Invest. 2018;128:1937–55.
Acknowledgements
We are grateful to the Doctors of Guizhou Medical University for their assistance with pancreatic cancer patient tissues collection.
Funding
This work was funded by the National Natural Science Foundation of China (No. 52273256) from KJ, Shenzhen Natural Science Fund (the Stable Support Plan Program (No. 20220810151804002)) from HL, Shenzhen Science and Technology Program (Grant No. RCBS20221008093107027) from ZH.
Author information
Authors and Affiliations
Contributions
HL: Methodology, investigation, and writing original draft. HL, ZH and KJ: Methodology, investigation, and validation. HL, ZZ, CW: Investigation, and validation. HL, ZZ, CW and JC: Validation and formal analysis and resources and data curation. HL, ZH and KJ: Conceptualization, resources, writing-review & Editing, supervision, funding acquisition, and project administration.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The approval of the Institutional Review Board at the Affiliated Hospital of Guizhou Medical University, Guizhou Medical University. All the animal studies were approved by the Shenzhen University Institutional Animal Care and Use Committee.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, H., Zhao, Z., Wu, C. et al. ZMYND8 promotes the Warburg effect and tumorigenesis through c-Myc activation in pancreatic cancer. Oncogene 44, 3083–3095 (2025). https://doi.org/10.1038/s41388-025-03483-0
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41388-025-03483-0