Abstract
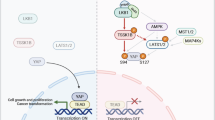
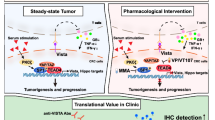
(Yes-associated protein 1) YAP1 is frequently activated in human prostate cancers (PCa), but the underlying regulatory mechanism remains elusive. Here, we identified a novel scaffold protein HOMER3 in PCa, that can promote YAP1 activity by disrupting LATS-YAP1 phosphorylation. Mechanistically, HOMER3 overexpression in PCa facilitates the SRC kinase to phosphorylate YAP1 accompanied by counteracting LATS1-mediated YAP1 inhibition, thereby maintaining high YAP1 nuclear localization and transcriptional activity. Accordingly, HOMER3 gain-of-function in PCa cells phenocopies the effect of YAP1 activation, including cell hyperproliferation in vitro and rapid tumor growth in vivo. Additionally, transcriptome analysis revealed that CD274 is consistently upregulated in HOMER3 overexpressing PCa cells and patients, which eventually contributed to an immunosuppressive phenotype. More importantly, blocking SRC kinase-mediated YAP1 activation improved the immunotherapy-insensitive phenotypes in PCa caused by HOMER3 overexpression. Taken together, our findings define a novel kinase-substrate interactive platform for HOMER3 to orchestrate YAP1 activity in PCa. Targeting SRC-YAP1 oncogenic axis provides new insights into the therapeutic potential for PCa patients carried HOMER3 overexpression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The datasets used in the current study are available from the corresponding author on reasonable request.
References
Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12–49.
Gong J, Kim DM, Freeman MR, Kim H, Ellis L, Smith B, et al. Genetic and biological drivers of prostate cancer disparities in Black men. Nat Rev Urol. 2024;21:274–89.
Corres-Mendizabal J, Zacchi F, Martín-Martín N, Mateo J, Carracedo A. Metastatic hormone-naïve prostate cancer: a distinct biological entity. Trends Cancer. 2024;10:825–41.
Bergengren O, Pekala KR, Matsoukas K, Fainberg J, Mungovan SF, Bratt O, et al. 2022 update on prostate cancer epidemiology and risk factors-a systematic review. Eur Urol. 2023;84:191–206.
Nguyen LT, Tretiakova MS, Silvis MR, Lucas J, Klezovitch O, Coleman I, et al. ERG activates the YAP1 transcriptional program and induces the development of age-related prostate tumors. Cancer Cell. 2015;27:797–808.
Li Q, Wang M, Hu Y, Zhao E, Li J, Ren L, et al. MYBL2 disrupts the Hippo-YAP pathway and confers castration resistance and metastatic potential in prostate cancer. Theranostics. 2021;11:5794–812.
Schirmer AU, Driver LM, Zhao MT, Wells CI, Pickett JE, O’Bryne SN, et al. Non-canonical role of Hippo tumor suppressor serine/threonine kinase 3 STK3 in prostate cancer. Mol Ther. 2022;30:485–500.
Gu Y, Wu S, Fan J, Meng Z, Gao G, Liu T, et al. CYLD regulates cell ferroptosis through Hippo/YAP signaling in prostate cancer progression. Cell Death Dis. 2024;15:1–12.
Wang K, Ma F, Arai S, Wang Y, Varkaris A, Poluben L, et al. WNT5a signaling through ROR2 activates the hippo pathway to suppress YAP1 activity and tumor growth. Cancer Res. 2023;83:1016–30.
Bainbridge A, Walker S, Smith J, Patterson K, Dutt A, Ng YM, et al. IKBKE activity enhances AR levels in advanced prostate cancer via modulation of the Hippo pathway. Nucleic Acids Res. 2020;48:5366–82.
Kuser-Abali G, Alptekin A, Lewis M, Garraway IP, Cinar B. YAP1 and AR interactions contribute to the switch from androgen-dependent to castration-resistant growth in prostate cancer. Nat Commun. 2015;6:8126.
Zhou P-J, Xue W, Peng J, Wang Y, Wei L, Yang Z, et al. Elevated expression of Par3 promotes prostate cancer metastasis by forming a Par3/aPKC/KIBRA complex and inactivating the hippo pathway. J Exp Clin Cancer Res. 2017;36:139.
Varzavand A, Hacker W, Ma D, Gibson-Corley K, Hawayek M, Tayh OJ, et al. α3β1 integrin suppresses prostate cancer metastasis via regulation of the hippo pathway. Cancer Res. 2016;76:6577–87.
Song H, Lu T, Han D, Zhang J, Gan L, Xu C, et al. YAP1 inhibition induces phenotype switching of cancer-associated fibroblasts to tumor suppressive in prostate cancer. Cancer Res. 2024;84:3728–42.
Xiao H, Du X, Tao Z, Jing N, Bao S, Gao W, et al. Taurine inhibits ferroptosis mediated by the crosstalk between tumor cells and tumor-associated macrophages in prostate cancer. Adv Sci. 2023;11:2303894.
Han H, Huang Z, Xu C, Seo G, An J, Yang B, et al. Functional annotation of the Hippo pathway somatic mutations in human cancers. Nat Commun. 2024;15:10106.
Luo J, Deng L, Zou H, Guo Y, Tong T, Huang M, et al. New insights into the ambivalent role of YAP/TAZ in human cancers. J Exp Clin Cancer Res. 2023;42:130.
Tong T, Huang M, Yan B, Lin B, Yu J, Teng Q, et al. Hippo signaling modulation and its biological implications in urological malignancies. Mol Asp Med. 2024;98:101280.
Zhong Z, Jiao Z, Yu F-X. The Hippo signaling pathway in development and regeneration. Cell Rep. 2024;43:113926.
Zhong Z, Meng Z, Yu F-X. Reconstructing the Hippo signaling network. Sci Bull. 2023;68:2307–10.
Xiong Y, Zhang X, Zhu J, Zhang Y, Pan Y, Wu Y, et al. Collagen I-DDR1 signaling promotes hepatocellular carcinoma cell stemness via Hippo signaling repression. Cell Death Differ. 2023;30:1648–65.
Pulkkinen HH, Kiema M, Lappalainen JP, Toropainen A, Beter M, Tirronen A, et al. BMP6/TAZ-Hippo signaling modulates angiogenesis and endothelial cell response to VEGF. Angiogenesis. 2021;24:129–44.
Gu Y, Wang Y, Sha Z, He C, Zhu Y, Li J, et al. Transmembrane protein KIRREL1 regulates Hippo signaling via a feedback loop and represents a therapeutic target in YAP/TAZ-active cancers. Cell Rep. 2022;40:111296.
Su D, Li Y, Zhang W, Gao H, Cheng Y, Hou Y et al. SPTAN1/NUMB axis senses cell density to restrain cell growth and oncogenesis through Hippo signaling. J Clin Invest. 2023;133. https://doi.org/10.1172/JCI168888.
Li F-L, Fu V, Liu G, Tang T, Konradi AW, Peng X, et al. Hippo pathway regulation by phosphatidylinositol transfer protein and phosphoinositides. Nat Chem Biol. 2022;18:1076–86.
Qi S, Zhu Y, Liu X, Li P, Wang Y, Zeng Y, et al. WWC proteins mediate LATS1/2 activation by Hippo kinases and imply a tumor suppression strategy. Mol Cell. 2022;82:1850–1864.e7.
Zhu R, Liu X, Zhang X, Zhong Z, Qi S, Jin R, et al. Gene therapy for diffuse pleural mesotheliomas in preclinical models by concurrent expression of NF2 and SuperHippo. Cell Rep. Med. 2024;5:101763.
Xue S, Chen X, Qiu G, Liao H, Qiang Z, Zhang Z et al. CLK1 Activates YAP to Promote Intrahepatic Cholangiocarcinogenesis. Cancer Res. 2024. https://doi.org/10.1158/0008-5472.CAN-24-0147.
Liu Q, He L, Li S, Li F, Deng G, Huang X, et al. HOMER3 facilitates growth factor-mediated β-Catenin tyrosine phosphorylation and activation to promote metastasis in triple negative breast cancer. J Hematol OncolJ Hematol Oncol. 2021;14:6.
Chu Y, Dai E, Li Y, Han G, Pei G, Ingram DR, et al. Pan-cancer T cell atlas links a cellular stress response state to immunotherapy resistance. Nat Med. 2023;29:1550–62.
Lu K-H, Tounsi A, Shridhar N, Küblbeck G, Klevenz A, Prokosch S et al. Dickkopf-3 contributes to the regulation of anti-tumor immune responses by mesenchymal stem cells. Front Immunol. 2015;6. https://doi.org/10.3389/fimmu.2015.00645.
Fang X, Bogomolovas J, Trexler C, Chen J. The BAG3-dependent and -independent roles of cardiac small heat shock proteins. JCI Insight. 2019;4. https://doi.org/10.1172/jci.insight.126464.
ElTanbouly MA, Noelle RJ. Rethinking peripheral T cell tolerance: checkpoints across a T cell’s journey. Nat Rev Immunol. 2021;21:257–67.
Yakou MH, Ghilas S, Tran K, Liao Y, Afshar-Sterle S, Kumari A, et al. TCF-1 limits intraepithelial lymphocyte antitumor immunity in colorectal carcinoma. Sci Immunol. 2023;8:eadf2163.
Bian X, Wang W, Abudurexiti M, Zhang X, Ma W, Shi G, et al. Integration analysis of single-cell multi-omics reveals prostate cancer heterogeneity. Adv Sci Weinh Baden-Wurtt Ger. 2024;11:e2305724.
Chen S, Zhu G, Yang Y, Wang F, Xiao Y-T, Zhang N, et al. Single-cell analysis reveals transcriptomic remodellings in distinct cell types that contribute to human prostate cancer progression. Nat Cell Biol. 2021;23:87–98.
Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109–18.
Hayashi MK, Tang C, Verpelli C, Narayanan R, Stearns MH, Xu R-M, et al. The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell. 2009;137:159–71.
Shiraishi-Yamaguchi Y, Furuichi T. The Homer family proteins. Genome Biol. 2007;8:206.
Peixoto A, Ferreira D, Azevedo R, Freitas R, Fernandes E, Relvas-Santos M, et al. Glycoproteomics identifies HOMER3 as a potentially targetable biomarker triggered by hypoxia and glucose deprivation in bladder cancer. J Exp Clin Cancer Res. 2021;40:191.
Sun T, Song C, Zhao G, Feng S, Wei J, Zhang L, et al. HOMER3 promotes non-small cell lung cancer growth and metastasis primarily through GABPB1-mediated mitochondrial metabolism. Cell Death Dis. 2023;14:1–11.
Luo J, Zou H, Guo Y, Tong T, Ye L, Zhu C, et al. SRC kinase-mediated signaling pathways and targeted therapies in breast cancer. Breast Cancer Res. 2022;24:99.
Poh AR, Ernst M. Functional roles of SRC signaling in pancreatic cancer: recent insights provide novel therapeutic opportunities. Oncogene. 2023;42:1786–801.
Chessa TAM, Jung P, Anwar A, Suire S, Anderson KE, Barneda D, et al. PLEKHS1 drives PI3Ks and remodels pathway homeostasis in PTEN-null prostate. Mol Cell. 2023;83:2991–3009.e13.
Teng Y, Cai Y, Pi W, Gao L, Shay C. Augmentation of the anticancer activity of CYT997 in human prostate cancer by inhibiting Src activity. J Hematol OncolJ Hematol Oncol. 2017;10:118.
Moro L, Simoneschi D, Kurz E, Arbini AA, Jang S, Guaragnella N, et al. Epigenetic silencing of the ubiquitin ligase subunit FBXL7 impairs c-SRC degradation and promotes epithelial-to-mesenchymal transition and metastasis. Nat Cell Biol. 2020;22:1130–42.
Pu T, Wang J, Wei J, Zeng A, Zhang J, Chen J, et al. Stromal-derived MAOB promotes prostate cancer growth and progression. Sci Adv. 2024;10:eadi4935.
Sridaran D, Chouhan S, Mahajan K, Renganathan A, Weimholt C, Bhagwat S, et al. Inhibiting ACK1-mediated phosphorylation of C-terminal Src kinase counteracts prostate cancer immune checkpoint blockade resistance. Nat Commun. 2022;13:6929.
Li X-F, Selli C, Zhou H-L, Cao J, Wu S, Ma R-Y, et al. Macrophages promote anti-androgen resistance in prostate cancer bone disease. J Exp Med. 2023;220:e20221007.
Acknowledgements
mRNA-seq and scRNA-seq analysis was assisted by Guangzhou Genedenovo Biotechnology Co., Ltd. (Guangzhou, China). The Data analysis was performed using the OmicShare tools, a free online platform for data analysis (https://www.omicshare.com/tools).
Funding
The present study was funded by the National Natural Science Foundation of China (Grant No. 82272689 to Jun P and 82072901 to Peng L), Shenzhen Basic Science Research (JCYJ20190809164617205 to Jun P), the Sanming Project of Medicine in Shenzhen (SZSM202011011 to Jun P), the Shenzhen Medical Research Fund (A2302037 to Mengjun H), the Research Start-up Fund of Part-time PI, SAHSYSU (ZSQYJZPI202003 to Jun P), the Shenzhen Science and Technology Innovation Commission (JCYJ20210324120409026 to Peng L), and the Guangdong Basic and Applied Basic Research Foundation (Grant No. 2021A1515111052 to Jun P).
Author information
Authors and Affiliations
Contributions
Tongyu Tong and Peng Li developed the concept and designed this work. Tongyu Tong, Hanqi Lei, Mengjun Huang, Hailin Zou, Yibo Guo, Qiliang Teng and Fei Cao performed the experiments and carried out the data acquisition. Zheng Yang performed pathological analysis. Peng Li, Jun Pang and Mengjun Huang provided funding acquisition. Tongyu Tong, Hanqi Lei and Juan Luo performed data analysis. Tongyu Tong, Xuyin Dai, Peng Li and Jun Pang edited and revised the manuscript. All authors read and approved this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
Written informed consent was obtained from all patients and the study was approved by the Ethics Committee of the Seventh Affiliated Hospital, Sun Yat-sen University (KY-2024-069-02). Animal experiments were performed according to the Health Guide for the Care and Use of Laboratory Animals approved by the Institutional Animal Care and Use Committee of Sun Yat-Sen University (SYSU-IACUC-2024-002513). All methods were performed in accordance with relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tong, T., Lei, H., Huang, M. et al. HOMER3 orchestrates SRC-YAP1 activity that promotes tumor cell growth and antagonizes anti-tumor immunotherapy in prostate cancer. Oncogene 44, 3895–3908 (2025). https://doi.org/10.1038/s41388-025-03548-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41388-025-03548-0
This article is cited by
-
Targeting YAP1-CD70 axis potentiates the efficacy of anti-PD-1 therapy in prostate cancer
Biomarker Research (2026)