Abstract
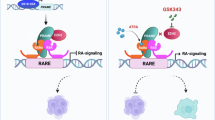
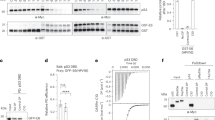
Preferentially expressed antigen in melanoma (PRAME), which is highly expressed in melanoma, is associated with tumor progression and malignancy. Notably, melanoma cells often exhibit inactivation of the tumor suppressor p53 despite carrying the wild-type p53 gene. Here, we investigated the functional interplay between PRAME and p53. Consistent with our analysis of human databases, PRAME overexpression promoted melanoma cell proliferation. Conversely, PRAME downregulation produced the opposite effects, accompanied by an increase in apoptosis. RNA sequencing revealed aberrant regulation of p53 target genes following PRAME depletion, which was further supported by reverse transcription-quantitative polymerase chain reaction and luciferase reporter assays. To explore the underlying mechanism, we isolated the PRAME protein complex and identified DBC1, an SIRT1 suppressor, as a component of the complex. Furthermore, we observed that PRAME promoted p53 deacetylation. The interaction of PRAME with DBC1 releases SIRT1 from DBC1, enabling SIRT1 activation and subsequent p53 deacetylation. The combination of PRAME depletion and SIRT1 inhibition can significantly promote the growth retardation of melanoma cells, as demonstrated by xenograft analysis in nude mice. Collectively, these findings suggest that the acquired elevation of the PRAME level during melanoma pathogenesis may suppress p53 pathways, thereby promoting tumor growth. We propose that PRAME silencing combined with the use of SIRT1 inhibitors is a promising therapeutic strategy for melanoma by restoring p53 activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The RNA-seq data from PRAME-depleted A375SM melanoma cells have been deposited in NCBI’s Gene Expression Omnibus (GEO) under accession number GSE306132.
References
Wagstaff W, Mwamba RN, Grullon K, Armstrong M, Zhao P, Hendren-Santiago B, et al. Melanoma: Molecular genetics, metastasis, targeted therapies, immunotherapies, and therapeutic resistance. Genes Dis. 2022;9:1608–23.
Yajima I, Kumasaka MY, Thang ND, Goto Y, Takeda K, Yamanoshita O, et al. RAS/RAF/MEK/ERK and PI3K/PTEN/AKT Signaling in Malignant Melanoma Progression and Therapy. Dermatol Res Pract. 2012;2012:354191.
Sabbah M, Najem A, Krayem M, Awada A, Journe F, Ghanem GE. RTK Inhibitors in Melanoma: From Bench to Bedside. Cancers. 2021;13:1685.
Davis EJ, Johnson DB, Sosman JA, Chandra S. Melanoma: What do all the mutations mean?. Cancer. 2018;124:3490–9.
Yang G, Rajadurai A, Tsao H. Recurrent patterns of dual RB and p53 pathway inactivation in melanoma. J Invest Dermatol. 2005;125:1242–51.
Eddy K, Chen S. Overcoming Immune Evasion in Melanoma. Int J Mol Sci. 2020;21:8984.
Lezcano C, Jungbluth AA, Nehal KS, Hollmann TJ, Busam KJ. PRAME Expression in Melanocytic Tumors. Am J Surg Pathol. 2018;42:1456–65.
Cascardi E, Cazzato G, Ingravallo G, Dellino M, Lupo C, Casatta N, et al. PReferentially Expressed Antigen in MElanoma (PRAME): preliminary communication on a translational tool able to early detect Oral Malignant Melanoma (OMM). J Cancer. 2023;14:628–33.
Oberthuer A, Hero B, Spitz R, Berthold F, Fischer M. The tumor-associated antigen PRAME is universally expressed in high-stage neuroblastoma and associated with poor outcome. Clin Cancer Res. 2003;10:4307–13.
Epping MT, Hart AA, Glas AM, Krijgsman O, Bernards R. PRAME expression and clinical outcome of breast cancer. Br J Cancer. 2008;99:398–403.
Shiseki M, Ishii M, Ohwashi M, Wang YH, Tanaka N, Osanai S, et al. High PRAME expression is associated with poor survival and early disease progression in myelodysplastic syndromes with a low bone marrow blast percentage. Leuk Lymphoma. 2021;62:2448–56.
Tanaka N, Wang YH, Shiseki M, Takanashi M, Motoji T. Inhibition of PRAME expression causes cell cycle arrest and apoptosis in leukemic cells. Leuk Res. 2011;35:1219–25.
Tan P, Zou C, Yong B, Han J, Zhang L, Su Q, et al. Expression and prognostic relevance of PRAME in primary osteosarcoma. Biochem Biophys Res Commun. 2012;419:801–8.
Zhu H, Wang J, Yin J, Lu B, Yang Q, Wan Y, et al. Downregulation of PRAME Suppresses Proliferation and Promotes Apoptosis in Hepatocellular Carcinoma Through the Activation of P53 Mediated Pathway. Cell Physiol Biochem. 2018;45:1121–35.
Epping MT, Wang L, Edel MJ, Carlée L, Hernandez M, Bernards R. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835–47.
Zhang W, Li L, Cai L, Liang Y, Xu J, Liu Y, et al. Tumor-associated antigen Prame targets tumor suppressor p14/ARF for degradation as the receptor protein of CRL2Prame complex. Cell Death Differ. 2021;28:1926–40.
Kurtenbach S, Sanchez MI, Kuznetsoff J, Rodriguez DA, Weich N, Dollar JJ, et al. PRAME induces genomic instability in uveal melanoma. Oncogene. 2024;43:555–65.
Xu Y, Yue Q, Wei H, Pan G. PRAME induces apoptosis and inhibits proliferation of leukemic cells in vitro and in vivo. Int J Clin Exp Pathol. 2015;8:14549–55.
Xu Y, Rong LJ, Meng SL, Hou FL, Zhang JH, Pan G. PRAME promotes in vitro leukemia cells death by regulating S100A4/p53 signaling. Eur Rev Med Pharm Sci. 2016;20:1057–63.
Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010;2:a001008.
Luca M, Lenzi R, Leejackson D, Gutman M, Fidler I, Bareli M. P53 mutations are infrequent and do not correlate with the metastatic potential of human-melanoma cells. Int J Oncol. 1993;3:19–22.
Weiss J, Heine M, Arden KC, Körner B, Pilch H, Herbst RA, et al. Mutation and expression of TP53 in malignant melanomas. Recent Results Cancer Res. 1995;139:137–54.
Houben R, Hesbacher S, Schmid CP, Kauczok CS, Flohr U, Haferkamp S, et al. High-level expression of wild-type p53 in melanoma cells is frequently associated with inactivity in p53 reporter gene assays. PLoS ONE. 2011;6:e22096.
Avery-Kiejda KA, Bowden NA, Croft AJ, Scurr LL, Kairupan CF, Ashton KA, et al. P53 in human melanoma fails to regulate target genes associated with apoptosis and the cell cycle and may contribute to proliferation. BMC Cancer. 2011;11:203.
Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9:402–12.
Dai C, Gu W. p53 post-translational modification: deregulated in tumorigenesis. Mol Med. 2010;16:528–36.
Yogosawa S, Yoshida K. Tumor suppressive role for kinases phosphorylating p53 in DNA damage-induced apoptosis. Cancer Sci. 2018;109:3376–82.
Xia Z, Kon N, Gu AP, Tavana O, Gu W. Deciphering the acetylation code of p53 in transcription regulation and tumor suppression. Oncogene. 2022;41:3039–50.
Kim EJ, Kho JH, Kang MR, Um SJ. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol Cell. 2007;28:277–90.
Kim JE, Chen J, Lou Z. DBC1 is a negative regulator of SIRT1. Nature. 2008;451:583–6.
Zhao W, Kruse JP, Tang Y, Jung SY, Qin J, Gu W. Negative regulation of the deacetylase SIRT1 by DBC1. Nature. 2008;451:587–90.
Lee YK, Park UH, Kim EJ, Hwang JT, Jeong JC, Um SJ. Tumor antigen PRAME is up-regulated by MZF1 in cooperation with DNA hypomethylation in melanoma cells. Cancer Lett. 2017;403:144–51.
Jeon D, Kim N, Um SJ. BET Inhibitors Induce p53-Independent Growth Arrest in HCT116 Cells via Epigenetic Control of the E2F1/c-MYC Axis. Biol Pharm Bull. 2032;46:12–8.
Lu M, Breyssens H, Salter V, Zhong S, Hu Y, Baer C, et al. Restoring p53 function in human melanoma cells by inhibiting MDM2 and cyclin B1/CDK1-phosphorylated nuclear iASPP. Cancer Cell. 2013;23:618–33.
Wadelin FR, Fulton J, Collins HM, Tertipis N, Bottley A, Spriggs KA, et al. PRAME is a golgi-targeted protein that associates with the Elongin BC complex and is upregulated by interferon-gamma and bacterial PAMPs. PLoS ONE. 2013;8:e58052.
Kang H, Suh JY, Jung YS, Jung JW, Kim MK, Chung JH. Peptide switch is essential for Sirt1 deacetylase activity. Mol Cell. 2011;44:203–13.
Kim HJ, Moon SJ, Kim JH. Mechanistic insights into the dual role of CCAR2/DBC1 in cancer. Exp Mol Med. 2023;55:1691–701.
Ohanna M, Bonet C, Bille K, Allegra M, Davidson I, Bahadoran P, et al. SIRT1 promotes proliferation and inhibits the senescence-like phenotype in human melanoma cells. Oncotarget. 2014;5:2085–95.
Wilking MJ, Singh C, Nihal M, Zhong W, Ahmad N. SIRT1 deacetylase is overexpressed in human melanoma and its small molecule inhibition imparts anti-proliferative response via p53 activation. Arch Biochem Biophys. 2014;563:94–100.
Saunderson EA, Encabo HH, Devis J, Rouault-Pierre K, Piganeau M, Bell CG, et al. CRISPR/dCas9 DNA methylation editing is heritable during human hematopoiesis and shapes immune progeny. Proc Natl Acad Sci USA. 2023;120:e2300224120.
Harshita, Harish V, Lad Upendra S, Mohd S, Singh SK, Agrawal P, et al. Next-gen cancer treatment: nanotechnology-driven siRNA delivery solutions. Assay Drug Dev Technol. 2025;23:115–28.
Zhang J, Chen C, Chen X, Liao K, Li F, Song X, et al. Linker-free PROTACs efficiently induce the degradation of oncoproteins. Nat Commun. 2025;16:4794.
Alonso MR, Grinyó-Escuer A, Duro-Sánchez S, Rius-Ruiz I, Bort-Brusca M, Escorihuela M, et al. Generation of chimeric antigen receptor T cells targeting p95HER2 in solid tumors. Nat Commun. 2024;15:9589.
Krzysiak TC, Choi YJ, Kim YJ, Yang Y, DeHaven C, Thompson L, et al. Inhibitory protein-protein interactions of the SIRT1 deacetylase are choreographed by post-translational modification. Protein Sci. 2024;33:e4938.
Kewitz S, Staege MS. Knock-down of PRAME increases retinoic acid signaling and cytotoxic drug sensitivity of Hodgkin lymphoma cells. PLoS ONE. 2013;8:e55897.
Yan H, Zhao RM, Wang ZJ, Zhao FR, Wang SL. Knockdown of PRAME enhances adriamycin-induced apoptosis in chronic myeloid leukemia cells. Eur Rev Med Pharm Sci. 2015;19:4827–34.
Lu M, Miller P, Lu X. Restoring the tumour suppressive function of p53 as a parallel strategy in melanoma therapy. FEBS Lett. 2014;588:2616–21.
Sasaki T, Gan EC, Wakeham A, Kornbluth S, Mak TW, Okada H. HLA-B-associated transcript 3 (Bat3)/Scythe is essential for p300-mediated acetylation of p53. Genes Dev. 2007;21:848–61.
Amson R, Pece S, Lespagnol A, Vyas R, Mazzarol G, Tosoni D, et al. Reciprocal repression between P53 and TCTP. Nat Med. 2011;18:91–9.
Jiang P, Du W, Mancuso A, Wellen KE, Yang X. Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence. Nature. 2013;493:689–93.
Wang C, Yang Y, Wu X, Li J, Liu K, Fang D, et al. Reciprocal modulation of long noncoding RNA EMS and p53 regulates tumorigenesis. Proc Natl Acad Sci USA. 2022;119:e2111409119.
Fenouille N, Puissant A, Tichet M, Zimniak G, Abbe P, Mallavialle A, et al. SPARC functions as an anti-stress factor by inactivating p53 through Akt-mediated MDM2 phosphorylation to promote melanoma cell survival. Oncogene. 2011;30:4887–900.
Gembarska A, Luciani F, Fedele C, Russell EA, Dewaele M, Villar S, et al. MDM4 is a key therapeutic target in cutaneous melanoma. Nat Med. 2012;18:1239–47.
Acknowledgements
We thank Dr. Bert Vogelstein (Howard Hughes Medical Institute, Johns Hopkins Oncology Center) for providing p21WAF1 and MDM2 promoter-luciferase reporter constructs.
Funding
This study was supported in part by the Basic Research Program through the National Research Foundation of Korea funded by the Ministry of Science and ICT (2022R1F1A1068623 to E.J.K.), by the National Research Foundation of Korea grant funded by the Korea government (MSIT) (RS-2025-00562288 to S.J.U.), and by Korea Basic Science Institute (National Research Facilities and Equipment Center) grant funded by the Ministry of Education (RS-2023-NF001356 to S.J.U.).
Author information
Authors and Affiliations
Contributions
Conceptualization, Y.K.L., H.H.H., H.Y., E.J.K., and S.J.U.; methodology, Y.K.L., H.H.H., U.H.P., and H.Y.; investigation, data curation, and visualization, Y.K.L., H.H.H.; writing, Y.K.L., H.H.H., E.J.K., and S.J.U.; supervision and funding acquisition, E.J.K. and S.J.U. All authors have read and agreed to submit this version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Sejong University (Approval No. SJ-20240111-02E1) and conducted in accordance with institutional guidelines.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, YK., Heo, H.H., Park, UH. et al. Tumor antigen PRAME promotes melanoma growth by inactivating p53 through the SIRT1-DBC1 axis. Oncogene 44, 4087–4099 (2025). https://doi.org/10.1038/s41388-025-03565-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41388-025-03565-z