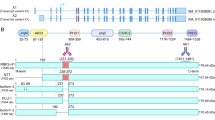
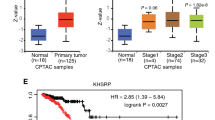
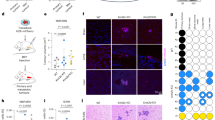
Abstract
25-Hydroxyvitamin D (25(OH)D), a metabolite of vitamin D, has demonstrated anticancer properties; however, the role of alternative splicing in mediating these effects remains poorly understood. In this study, we reveal for the first time that 25(OH)D exerts antitumor effects by promoting exon 13 skipping of KDM6A (KDM6A Δexon13), which suppresses the proliferation and stemness of breast cancer cells and lacks H3K27 demethylase activity. Mechanistically, CUT&Tag and RNA-seq analyses demonstrated that KDM6A Δexon13 induces the accumulation of H3K27me3 at the promoter region of TRAP1, thereby inhibiting its transcription. Consequently, the downregulation of TRAP1 reduces Smad2/3 phosphorylation. Furthermore, KHDRBS3 was identified as the splicing factor of KDM6A Δexon13 and was regulated by 25(OH)D. Notably, 25(OH)D exhibited a synergistic effect with GSK-J4, a specific inhibitor of KDM6A, in suppressing breast cancer cell growth. Collectively, our findings uncover a novel anticancer mechanism of 25(OH)D, highlight the critical role of KDM6A Δexon13 in breast cancer progression, and provide further evidence supporting the correction of 25(OH)D deficiency in breast cancer patients.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The RNA-seq and the CUT&Tag data have been deposited in the NCBI (https://www.ncbi.nlm.nih.gov/) Sequence Read Archive (SRA) database under accession number PRJNA1216307.
References
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. 2024;74:229–63.
Zheng W, Peng W, Qian F, Zhang M, Duan B, Fan Z, et al. Vitamin D suppresses CD133+/CD44 + cancer stem cell stemness by inhibiting NF-kappaB signaling and reducing NLRP3 expression in triple-negative breast cancer. Cancer Chemother Pharm. 2024;94:67–78.
Kuznia S, Zhu A, Akutsu T, Buring JE, Camargo CA, Jr., Cook NR, et al. Efficacy of vitamin D(3) supplementation on cancer mortality: Systematic review and individual patient data meta-analysis of randomised controlled trials. Ageing Res Rev. 2023;87:101923.
Kim Y, Chang Y, Cho Y, Chang J, Kim K, Park D-I, et al. Serum 25-hydroxyvitamin D levels and risk of colorectal cancer: an age-stratified analysis. Gastroenterology. 2023;165:920–31.
Zemlin C, Altmayer L, Stuhlert C, Schleicher JT, Wormann C, Lang M, et al. Prevalence and Relevance of Vitamin D Deficiency in Newly Diagnosed Breast Cancer Patients: A Pilot Study. Nutrients, 2023;15:1450.
Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342–57.
Townsend K, Banwell CM, Guy M, Colston KW, Mansi JL, Stewart PM, et al. Autocrine metabolism of vitamin D in normal and malignant breast tissu e. Clin Cancer Res. 2005;11:3579–86.
Aggarwal A, Feldman D, Feldman BJ. Identification of tumor-autonomous and indirect effects of vitamin D action that inhibit breast cancer growth and tumor progression. J Steroid Biochem Mol Biol. 2018;177:155–8.
Shan NL, Wahler J, Lee HJ, Bak MJ, Gupta SD, Maehr H, et al. Vitamin D compounds inhibit cancer stem-like cells and induce differentiation in triple negative breast cancer. J Steroid Biochem Mol Biol. 2017;173:122–9.
Xie SP, Pirianov G, Colston KW. Vitamin D analogues suppress IGF-I signalling and promote apoptosis in breast cancer cells. Eur J Cancer. 1999;35:1717–23.
Carlberg C, Muñoz A. An update on vitamin D signaling and cancer. Semin Cancer Biol. 2022;79:217–30.
Marasco LE, Kornblihtt AR. The physiology of alternative splicing. Nat Rev Mol Cell Biol. 2022;24:242–54.
Bonnal SC, López-Oreja I, Valcárcel J. Roles and mechanisms of alternative splicing in cancer — implications for care. Nat Rev Clin Oncol. 2020;17:457–74.
Castagnoli L, Iezzi M, Ghedini GC, Ciravolo V, Marzano G, Lamolinara A, et al. Activated d16HER2 Homodimers and SRC Kinase Mediate Optimal Efficacy for Trastuzumab. Cancer Res. 2014;74:6248–59.
Castagnoli L, Ghedini GC, Koschorke A, Triulzi T, Dugo M, Gasparini P, et al. Pathobiological implications of the d16HER2 splice variant for stemnes s and aggressiveness of HER2-positive breast cancer. Oncogene. 2017;36:1721–32.
Lee MG, Villa R, Trojer P, Norman J, Yan K-P, Reinberg D, et al. Demethylation of H3K27 regulates polycomb recruitment and H2A ubiquiti nation. Science. 2007;318:447–50.
Simon JA, Kingston RE. Occupying chromatin: polycomb mechanisms for getting to genomic targets, stopping transcriptional traffic, and staying put. Mol Cell. 2013;49:808–24.
Hosogane M, Funayama R, Shirota M, Nakayama K. Lack of transcription triggers H3K27me3 accumulation in the gene body. Cell Rep. 2016;16:696–706.
Shirai CL, Ley JN, White BS, Kim S, Tibbitts J, Shao J, et al. Mutant U2AF1 Expression Alters Hematopoiesis and Pre-mRNA Splicing In Vivo. Cancer Cell. 2015;27:631–43.
Wan X, Shi W, Ma L, Wang L, Zheng R, He J, et al. A 3′‐pre‐tRNA‐derived small RNA tRF‐1‐Ser regulated by 25(OH)D promotes proliferation and stemness by inhibiting the function of MBNL1 in breast cancer. Clin Transl Med. 2024;14:e1681.
Mahmood N, Arakelian A, Muller WJ, Szyf M, Rabbani SA. An enhanced chemopreventive effect of methyl donor S-adenosylmethionine in combination with 25-hydroxyvitamin D in blocking mammary tumor growth and metastasis. Bone Res. 2020;8:28.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30.
Seiler M, Peng S, Agrawal AA, Palacino J, Teng T, Zhu P, et al. Somatic mutational landscape of splicing factor genes and their functional consequences across 33 cancer types. Cell Rep. 2018;23:282–96.e4.
Masgras I, Laquatra C, Cannino G, Serapian SA, Colombo G, Rasola A. The molecular chaperone TRAP1 in cancer: from the basics of biology to pharmacological targeting. Semin Cancer Biol. 2021;76:45–53.
Zhang B, Wang J, Huang Z, Wei P, Liu Y, Hao J, et al. Aberrantly upregulated TRAP1 is required for tumorigenesis of breast c ancer. Oncotarget. 2015;6:44495–508.
Sisinni L, Maddalena F, Lettini G, Condelli V, Matassa DS, Esposito F, et al. TRAP1 role in endoplasmic reticulum stress protection favors resistance to anthracyclins in breast carcinoma cells. Int J Oncol. 2014;44:573–82.
Wengert LA, Backe SJ, Bourboulia D, Mollapour M, Woodford MR. TRAP1 chaperones the metabolic switch in cancer. Biomolecules. 2022;12:786.
Song C, Xu X, Wu Y, Ji B, Zhou X, Qin L. Study of the mechanism underlying hsa-miR338-3p downregulation to promote fibrosis of the synovial tissue in osteoarthritis patients. Mol Biol Rep. 2018;46:627–37.
Jie P, Wu Y, Song C, Cheng Y, Liu Y, Chen K. Mechanism of Nrf2/miR338-3p/TRAP-1 pathway involved in hyperactivation of synovial fibroblasts in patients with osteoarthritis. Heliyon. 2023;9:e21412.
Matsumoto Y, Itou J, Sato F, Toi M. SALL4 - KHDRBS3 network enhances stemness by modulating CD44 splicing in basal-like breast cancer. Cancer Med. 2018;7:454–62.
Dalpatraj N, Naik A, Thakur N. GSK-J4: an H3K27 histone demethylase inhibitor, as a potential anti-cancer agent. Int J Cancer. 2023;153:1130–8.
Ben-Eltriki M, Deb S, Guns EST. 1α,25-Dihydroxyvitamin D3 synergistically enhances anticancer effects of ginsenoside Rh2 in human prostate cancer cells. J Steroid Biochem Mol Biol. 2021;209:105828.
Zheng C, Zhang B, Li Y, Liu K, Wei W, Liang S, et al. Donafenib and GSK-J4 synergistically induce ferroptosis in liver cancer by upregulating HMOX1 expression. Adv Sci. 2023;10:e2206798.
Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33–44.
Rainville C, Khan Y, Tisman G. Triple negative breast cancer patients presenting with low serum vitamin D levels: a case series. Cases J. 2009;2:8390.
Lope V, Castello A, Mena-Bravo A, Amiano P, Aragones N, Fernandez-Villa T, et al. Serum 25-hydroxyvitamin D and breast cancer risk by pathological subtype (MCC-Spain). J Steroid Biochem Mol Biol. 2018;182:4–13.
Kim HJ, Lee YM, Ko BS, Lee JW, Yu JH, Son BH, et al. Vitamin D deficiency is correlated with poor outcomes in patients with luminal-type breast cancer. Ann Surg Oncol. 2011;18:1830–6.
Cristobo I, Larriba MJ, Ríos Vdl, García F, Muñoz A, Casal JI. Proteomic analysis of 1α,25-Dihydroxyvitamin D3 action on human colon cancer cells reveals a link to splicing regulation. J Proteom. 2011;75:384–97.
Nurminen V, Neme A, Seuter S, Carlberg C. The impact of the vitamin D-modulated epigenome on VDR target gene regulation. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mech. 2018;1861:697–705.
Yan N, Xu L, Wu X, Zhang L, Fei X, Cao Y, et al. GSKJ4, an H3K27me3 demethylase inhibitor, effectively suppresses the breast cancer stem cells. Exp Cell Res. 2017;359:405–14.
Lan J, Wei G, Liu J, Yang F, Sun R, Lu H. Chemotherapy-induced adenosine A2B receptor expression mediates epigenetic regulation of pluripotency factors and promotes breast cancer stemness. Theranostics. 2022;12:2598–612.
Lu H, Xie Y, Tran L, Lan J, Yang Y, Murugan NL, et al. Chemotherapy-induced S100A10 recruits KDM6A to facilitate OCT4-mediated breast cancer stemness. J Clin Investig. 2020;130:4607–23.
Yang Y, Chen C, Zuo Q, Lu H, Salman S, Lyu Y, et al. NARF is a hypoxia-induced coactivator for OCT4-mediated breast cancer stem cell specification. Sci Adv. 2022;8:eabo5000.
Fotouhi O, Nizamuddin S, Falk S, Schilling O, Knüchel-Clarke R, Biniossek ML, et al. Alternative mRNA Splicing Controls the Functions of the Histone H3K27 Demethylase UTX/KDM6A. Cancers. 2023;15:3117
Lettini G, Sisinni L, Condelli V, Matassa DS, Simeon V, Maddalena F, et al. TRAP1 regulates stemness through Wnt/β-catenin pathway in human colorectal carcinoma. Cell Death Differ. 2016;23:1792–803.
Bhreathnach U, Griffin B, Brennan E, Ewart L, Higgins D, Murphy M. Profibrotic IHG-1 complexes with renal disease associated HSPA5 and TRAP1 in mitochondria. Biochimica et Biophysica Acta (BBA) - Mol Basis Dis. 2017;1863:896–906.
Chen J-f, He Q, Dai M-h, Kong W. HSP75 inhibits TGF-β1-induced apoptosis by targeting mitochondria in human renal proximal tubular epithelial cells. Biochem Biophys Res Commun. 2019;515:64–71.
Takemoto K, Miyata S, Takamura H, Katayama T, Tohyama M. Mitochondrial TRAP1 regulates the unfolded protein response in the endoplasmic reticulum. Neurochem Int. 2011;58:880–7.
Anczuków O, Krainer AR. Splicing-factor alterations in cancers. Rna. 2016;22:1285–301.
Ukai S, Honma R, Sakamoto N, Yamamoto Y, Pham QT, Harada K, et al. Molecular biological analysis of 5-FU-resistant gastric cancer organoids; KHDRBS3 contributes to the attainment of features of cancer stem cell. Oncogene. 2020;39:7265–78.
Somrit K, Krobthong S, Yingchutrakul Y, Phueakphud N, Wongtrakoongate P, Komyod W. KHDRBS3 facilitates self-renewal and temozolomide resistance of glioblastoma cell lines. Life Sci. 2024;358:123132
Zhao M, Zhang Y, Li L, Liu X, Zhou W, Wang C, et al. KHDRBS3 accelerates glycolysis and promotes malignancy of hepatocellular carcinoma via upregulating 14-3-3ζ. Cancer Cell Int. 2023;23:244
Ukai S, Sakamoto N, Taniyama D, Harada K, Honma R, Maruyama R, et al. KHDRBS3 promotes multi-drug resistance and anchorage-independent growth in colorectal cancer. Cancer Sci. 2021;112:1196–208.
Bradley RK, Anczuków O. RNA splicing dysregulation and the hallmarks of cancer. Nat Rev Cancer. 2023;23:135–55.
Han T, Goralski M, Gaskill N, Capota E, Kim J, Ting TC, et al. Anticancer sulfonamides target splicing by inducing RBM39 degradation via recruitment to DCAF15. Science. 2017;356:eaal3755.
Abu-Hanna J, Patel JA, Anastasakis E, Cohen R, Clapp LH, Loizidou M, et al. Therapeutic potential of inhibiting histone 3 lysine 27 demethylases: a review of the literature. Clin Epigenetics. 2022;14:98
Kruidenier L, Chung C-w, Cheng Z, Liddle J, Che K, Joberty G, et al. A selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response. Nature. 2012;488:404–8.
Acknowledgements
This research was partially supported by the Chinese Society of Clinical Oncology Foundation (Y-HR2020MS-0421), Jiangsu Province Elderly Health Research Project (LKM2022033, LKM2023023), Wu Jieping Medical Foundation Special Fund for Clinical Research (320.6750.2023-11-29), China Breast Surgery Young Physician Research Award Advantage Support Program (2020-CHPASLP-01), National Natural Science Foundation of China (Grant No. 82503790).
Author information
Authors and Affiliations
Contributions
LJM, XYS, and LXW performed the experiments and analyzed the data. XYW provided technical support for in vitro experiments, and we would like to express our special gratitude for his significant contributions. XL and RZ accomplished clinical sample and data collection. LJM, LX, and JW conceived the study. LX, JW, and XMZ supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All BC tissue samples were obtained from the Department of Breast Disease of The First Affiliated Hospital of Nanjing Medical University. The collection of clinical samples was approved by the Ethics Committee of Jiangsu Province Hospital (ethics review number: 2023-SR-977). All participants provided written informed consent prior to sample collection. All animal experiments were approved by the Institutional Animal Care and Use Committee of Nanjing Medical University (ethics review number: IACUC-2309011). All procedures were carried out in accordance with institutional and national ethical guidelines for animal research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, L., Sheng, X., Wang, L. et al. KDM6A alternative splicing induced by 25(OH)D inhibits breast cancer cell stemness through repressing TRAP1 transcription. Oncogene (2026). https://doi.org/10.1038/s41388-026-03694-z
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41388-026-03694-z