Abstract
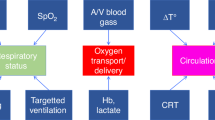
Monitoring of cerebral oxygenation (rScO2) with near-infrared spectroscopy (NIRS) is a feasible noninvasive bedside technique in the NICU. This review discusses the possible neuroprotective role of “pattern recognition” of NIRS-derived rScO2 in preterm neonates with regard to the prevention of severe intraventricular hemorrhage and hypoxia/hyperoxia-related white matter injury. This neuroprotective role of rScO2 monitoring is discussed as a modality to aid in the early detection of cerebral oxygenation conditions predisposing to these complications. Practical guidelines are provided concerning management of abnormal rScO2 patterns as well as a brief discussion concerning the need for international consensus and the legal aspects associated with the introduction of a new NICU bedside monitoring strategy.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Change history
06 August 2018
The original version of this article contained an error in the legend of Fig. 3, which incorrectly read:
Figure 3. a The patterns of arterial saturation (SaO2; orange), and rScO2 (blue) and mean arterial blood pressure (MABP; red) of an extremely preterm infant on postnatal day 1. The initial rScO2 values were very low (red box). These low values seemed to be associated with PaCO2 values below 30 mmHg (brown squares; starting at 24 mmHg. SaO2 and MABPs values were always normal. When PaCO2 values increased above values of 30 mmHg (brown arrow) the rScO2 increased and eventually normalized. b The patterns of rScO2 (blue) and mean arterial blood pressure (MABP; red) of a very preterm girl, starting on postnatal day 1, was especially marked by a steep decrease in cerebral oxygenation (rScO2; red box) to very low values (<40%). Echocardiographic investigation early on postnatal day 2 revealed a hemodynamically significant ductus arteriosus. Subsequent ductal closure with indomethacin (2 courses) was followed by normalization of cerebral oxygenation. c The patterns of heart rate (HR), arterial saturation (SaO2) and rScO2 (red box) in a preterm neonate.with severe anemia. The rather low rScO2 recovered following packed red blood cell transfusion (courtesy Prof. Gunnar Naulaers, UZ Leuven).
This has been corrected in both the PDF and HTML versions of the article.
References
Van Bel, F., Lemmers, P. & Naulaers, G. Monitoring neonatal regional cerebral oxygen saturation in clinical practice: value and pitfalls. Neonatology 94, 237–244 (2008).
Dix, L. M. L., van Bel, F. & Lemmers, P. M. A. Monitoring cerebral oxygenation in neonates: an update. Front. Pediatr. 5, 46 (2017).
Wintermark, P., Hansen, A., Warfield, S. K., Dukhovny, D. & Soul, J. S. Near-infrared spectroscopy versus magnetic resonance imaging to study brain perfusion in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neuroimage 85, 287–293 (2014).
Alderliesten, T. et al. Brain oxygen saturation assessment in neonates using T2-prepared blood imaging of oxygen saturation and near-infrared spectroscopy. J. Cereb. Blood Flow Metab. 37, 902–913 (2016).
Tsuji, M. et al. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics 106, 625–632 (2000).
Brady, K. et al. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke 41, 1951–1956 (2010).
Hyttel-Sorensen, S. et al. Cerebral near infrared spectroscopy oximetry in extremely preterm infants: phase II randomized clinical trial. BMJ 350, g7635 (2015).
Alderliesten, T. et al. Reference values of regional cerebral oxygen saturation during the first 3 days of life in preterm neonates. Pediatr. Res. 79, 55–64 (2016).
Plomgaard, A. M. et al. Early biomarkers of brain injury and cerebral hypo- and hyperoxia in the SafeBoosC II trial. PLoS ONE 12, e0173440 (2017).
Verhagen, E. A. et al. Cerebral oxygenation is associated with neurodevelopmental outcome of preterm children at age 2 to 3 years. Dev. Med. Child Neurol. 57, 449–455 (2015).
Sorensen, L. C. & Greisen, G. Precision of measurement of cerebral tissue oxygenation index using near-infrared spectroscopy in preterm neonates. J. Biomed. Opt. 11, 054005 (2006).
Greisen, G., Andrese, B., Plomgaard, A. M. & Hyttel-Sorensen, S. Cerebral oximetry in preterm infants: an agenda for research with a clear clinical goal. Neurophotonics 3, 031407–1–6 (2016).
Korcek, P., Stranak, Z., Sirc, J. & Naulaers, G. The role of near-infrared spectroscopy monitoring in preterm infants. J. Perinatol. 37, 1070–1077 (2017).
Mintzer, J. P., Parvez, B., Chelala, M., Alpan, G. & LaGamma, E. F. Quiescent variability of cerebral, renal, and splanchnic regional tissue oxygenation in very low birth weight neonates. J. Neonatal Perinatal Med. 7, 89–100 (2014).
Dix, L. M., van Bel, F., Baerts, W. & Lemmers, P. M. Comparing near-infrared spectroscopy devices and their sensors for monitoring regional cerebral oxygen saturation in the neonate. Pediatr. Res. 74, 557–563 (2013).
Vohr, B. R. et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National institute of Child Health and Human Development Neonatal Research Network, 1993-1994. Pediatrics 105, 1216–1226 (2000).
Brown, N. C. et al. Neurobehavior at term and white and gray matter abnormalities in very preterm infants. J. Pediatr. 155, 32–38 (2009).
Volpe, J. J. Neurology of the Newborn 6th edn, 325–698 (Elsevier, Philadephia, PA, 2018).
Krediet, T. G., Kavelaars, A., Vreman, H. I., Heijnen, C. J. & van Bel, F. Respiratory distress syndrome-associated inflammation is related to early but not late peri/intraventricular hemorrhage in preterm infants. J. Pediatr. 148, 740–746 (2006).
Ballabh, P. Pathogenesis and prevention of intraventricular hemorrhage. Clin. Perinatol. 41, 47–67 (2014).
Alderliesten, T. et al. Cerebral oxygenation, extraction, and autoregulation in very preterm infants who develop peri-intraventricular hemorrhage. J. Pediatr. 162, 698–704 (2013).
Perlman, J. M., McMenamin, J. B. & Volpe, J. J. Fluctuating cerebral blood-flow velocity in respiratory-distress syndrome. Relation to the development of intraventricular hemorrhage. N. Engl. J. Med. 309, 204–209 (1983).
Dix, L. M. L. et al. Carbon dioxide fluctuations are associated with changes in cerebral oxygenation and electrical activity in infants born preterm. J. Pediatr. 187, 66–72.e1 (2017).
Bonestroo, H. J. C., Lemmers, P. M. A., Baerts, W. & van Bel, F. Effect of antihypotensive treatment on cerebral oxygenation of preterm infants without PDA. Pediatrics 128, e1502–e1510 (2011).
Alderliesten, T. et al. Hypotension in preterm neonates: low blood pressure alone does not affect neurodevelopmental outcome. J. Pediatr. 164, 986–991 (2014).
Inder, T. E., Wells, S. J., Mogridge, N. B., Spencer, C. & Volpe, J. J. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J. Pediatr. 143, 171–179 (2003).
Volpe, J. J. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet 8, 110–124 (2009).
Billiards, S. S. et al. Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia. Brain Pathol. 18, 153–160 (2008).
Brouwer, M. J. et al. brain injury on term-equivalent age MRI in relation to perinatal factors and neurodevelopmental outcome at two years. PLoS ONE 12, e0177128 (2017).
Favrais, G. et al. Systemic inflammation disrupts the developmental program of white matter. Ann. Neurol. 70, 550–565 (2011).
Shankaran, S. et al. Cumulative index of exposure to hypocarbia and hyperoxia as risk factors for periventricular leukomalacia in low birth weight infants. Pediatrics 118, 1654–1659 (2006).
Hatzidaki, E. et al. Risk factors for periventricular leukomalacia. Acta Obstet. Gynecol. Scand. 88, 110–115 (2009).
Martin, C. G., Snider, A. R., Katz, S. M., Peabody, J. L. & Brady, J. P. Abnormal cerebral blood flow patterns in preterm infants with a large patent ductus arteriosus. J. Pediatr. 101, 587–593 (1982).
Lemmers, P. M. A. et al. Patent ductus arteriosus and brain volume. Pediatrics 137, e20153090 (2016).
Andersen, C. C., Karayil, S. M., Hodyl, N. A. & Stark, M. J. Early red cell transfusion favourably alters cerebral oxygen extraction in very preterm newborns. Arch. Dis. Child. Fetal Neonatal Ed. 100, F433–F435 (2015).
Mintzer, J. P., Parvez, B., Chelala, M., Alpan, G. & LaGamma, E. F. Monitoring regional tissue extraction in neonates 1250g helps identify transfusion tresholds independent of hematocrit. J. Neonatal Perinatal Med 7, 89–100 (2014).
Van Hoften, J. C., Verhagen, E. A., Keating, P., ter Horst, H. J. & Bos, A. F. Cerebral tissue oxygen saturation and extraction in preterm infants before and after blood transfusion. Arch. Dis. Child. Fetal Neonatal Ed. 95, F352–F358 (2010).
Vanderhaegen, J., Vanhaesebrouck, S., Vanhole, C., Casaer, P. & Naulaers, G. The effect of glycaemia on the cerebral oxygenation in very low birthweight infants as measured by near-infrared spectroscopy. Adv. Exp. Med. Biol. 662, 461–466 (2010).
Kochan, M. et al. Changes in cerebral oxygenation in preterm infants with progressive posthemorrhagic ventricular dilatation. Pediatr. Neurol. 73, 57–63 (2017).
Greissen, G. & Vannucci, R. C. Is periventricular leucomalacia a result of hypoxic-ischaemic injury? Hypocapnia and the preterm brain. Biol. Neonate 79, 194–200 (2001).
Benitz, W. E. Treatment of persistent patent ductus arteriosus in preterm infants: time to accept the null hypothesis? J. Pediatr. 30, 241–252 (2010).
Noori, S. Patent ductus arteriosus in the preterm infant: to treat or not to treat?. J. Perinatol. 30(Suppl.), S31–S37 (2010).
Hou, X. et al. Research on the relationship between brain anoxia at different regional oxygen saturations and brain damage using near-infrared spectroscopy. Physiol. Meas. 28, 1251–1265 (2007).
Kurth, C. D., McCann, J. C., Wu, J., Miles, L. & Loepke, A. W. Cerebral oxygen saturation-time threshold for hypoxic-ischemic injury in piglets. Anesth. Analg. 108, 1268–1277 (2009).
Dent, C. L. et al. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J. Thorac. Cardiovasc. Surg. 131, 190–197 (2006).
Kusaka, T. et al. Relationship between cerebral oxygenation and phosphorylation potential during secondary energy failure in hypoxic-ischemic newborn piglets. Pediatr. Res. 65, 317–322 (2009).
Limperopoulos, C. et al. Cerebellar hemorrhage in the preterm infant: ultrasonographic findings and risk factors. Pediatrics 116, 717–724 (2005).
Padilla, N., Alexandrou, G., Blennow, M., Lagercrantz, H. & Aden, U. Brain growth gains and losses in extremely preterm infanst at term. Cereb. Cortex 25, 1897–1905 (2015).
Dani, C., Pratesi, S., Fontanelli, G., Barp, J. & Bertini, G. Blood transfuisons increase cerebral, splanchnic, and renal oxygenation in anemic preterm infants. Transfusion 50, 1220–1226 (2010).
Sandal, G. et al. Assessment of red blood cell transfusion and transfusion duration on cerebral and mesenteric oxygenation using near-infrared spectroscopy in preterm infants with symptomatic anemia. Transfusion 54, 1100–1105 (2014).
Soul, J. S., Eichenwald, E., Walter, G., Volpe, J. J. & du Plessis, A. J. CSF removal in infantile posthemorrhagic hydrocephalus results in significant improvement in cerebral hemodynamics. Pediatr. Res. 55, 872–876 (2004).
Pellicer, A. et al. The SafeBoosC phase II randomized clinical trial: a treatment guideline for targeted near-infrared-derived cerebral tissue oxygenation versus standard treatment in extremely preterm infants. Neonatology 104, 1271–1278 (2013).
Garvey, A. A. & Dempsey, E. M. Applications of near infrared spectroscopy in the neonate. Curr. Opin. Pediatr. 30, 209–215 (2018).
Andersen, C. C., Hodyl, N. A., Kirpalani, H. M. & Stark, M. J. A theoretical and practical approach to defining “adequate oxygenation” in the preterm newborn. Pediatrics 139, e20161117 (2017).
Acknowledgements
The authors thank Dr. Petra Lemmers and Dr. Willem Baerts for their critical reading of the manuscript and advice. No financial assistance was received in support of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
van Bel, F., Mintzer, J.P. Monitoring cerebral oxygenation of the immature brain: a neuroprotective strategy?. Pediatr Res 84, 159–164 (2018). https://doi.org/10.1038/s41390-018-0026-8
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-018-0026-8
This article is cited by
-
Wearable fiber-free optical sensor for continuous monitoring of neonatal cerebral blood flow and oxygenation
Pediatric Research (2024)
-
Skin-interfacing wearable biosensors for smart health monitoring of infants and neonates
Communications Materials (2024)
-
Impact of breast milk on cortical pain response in newborns during the heel prick procedure: a randomized controlled trial
Journal of Perinatology (2024)
-
Relationship of cerebral blood volume with arterial and venous flow velocities in extremely low-birth-weight infants
European Journal of Pediatrics (2023)
-
Biomarker und Neuromonitoring zur Entwicklungsprognose nach perinataler Hirnschädigung
Monatsschrift Kinderheilkunde (2022)