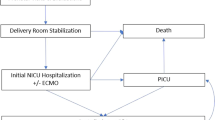
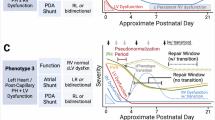
Abstract
Treatment modalities for neonates born with congenital diaphragmatic hernia (CDH) have greatly improved in recent times with a concomitant increase in survival. In 2008, CDH EURO consortium, a collaboration of a large volume of CDH centers in Western Europe, was established with a goal to standardize management and facilitate multicenter research. However, limited knowledge on long-term outcomes restricts the identification of optimal care pathways for CDH survivors in adolescence and adulthood. This review aimed to evaluate the current practice of long-term follow-up within the CDH EURO consortium centers, and to review the literature on long-term outcomes published from 2000 onward. Apart from having disease-specific morbidities, children with CDH are at risk for impaired neurodevelopmental problems and failure of educational attainments which may affect participation in society and the quality of life in later years. Thus, there is every reason to offer them long-term multidisciplinary follow-up programs. We discuss a proposed collaborative project using standardized clinical assessment and management plan (SCAMP) methodology to obtain uniform and standardized follow-up of CDH patients at an international level.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
American Academy of Pediatrics Section on Surgery, American Academy of Pediatrics Committee on Fetus and Newborn, Lally, K. P. & Engle, W. Postdischarge follow-up of infants with congenital diaphragmatic hernia. Pediatrics 121, 627–632 (2008).
Chiu, P. P. L. et al. The price of success in the management of congenital diaphragmatic hernia: is improved survival accompanied by an increase in long-term morbidity? J. Pediatr. Surg. 41, 888–892 (2006).
Van Den Hout, L. et al. Actual outcome in infants with congenital diaphragmatic hernia: the role of a standardized postnatal treatment protocol. Fetal Diagn. Ther. 29, 55–63 (2011).
Lund, D. P. et al. Congenital diaphragmatic hernia: the hidden morbidity. J. Pediatr. Surg. 29, 258–262 (1994).
Chiu, P. P. L. & Ijsselstijn, H. Morbidity and long-term follow-up in CDH patients. Eur. J. Pediatr. Surg. 22, 384–392 (2012).
Reiss, I. et al. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO consortium consensus. Neonatology 98, 354–364 (2010).
Snoek, K. G. et al. Conventional mechanical ventilation versus high-frequency oscillatory ventilation for congenital diaphragmatic hernia. A randomized clinical trial (The VICI-trial). Ann. Surg. 263, 867–874 (2016).
Snoek, K. G. et al. Standardized Postnatal Management of Infants with Congenital Diaphragmatic Hernia in Europe: The CDH EURO Consortium Consensus-2015 Update. Neonatology 110, 66–74 (2016).
Snoek, K. G. et al. Neurodevelopmental outcome in high-risk congenital diaphragmatic hernia patients: an appeal for international standardization. Neonatology 109, 14–21 (2015).
Rathod, R. H. et al. A novel approach to gathering and acting on relevant clinical information: SCAMPs. Congenit. Heart Dis. 5, 343–353 (2010).
Benoist, G. et al. Risk of readmission for wheezing during infancy in children with congenital diaphragmatic hernia. PLoS ONE 11, e0155556 (2016).
Davis, P. J. et al. Long-term outcome following extracorporeal membrane oxygenation for congenital diaphragmatic hernia: The UK experience. J. Pediatr. 144, 309–315 (2004).
Najaf T. A., Vachharajani A. J., Warner B. W. Follow up of children with congenital diaphragmatic hernia and development of a multidisciplinary care program. Inter. J. Pediatr. Neonatol. 16 (2013).
Basek, P. et al. The pulmonary outcome of long-term survivors after congenital diaphragmatic hernia repair. Swiss Med. Wkly 138, 173–179 (2008).
Rocha, G., Azevedo, I., Pinto, J. C. & Guimarães, H. Follow-up of the survivors of congenital diaphragmatic hernia. Early Hum. Dev. 88, 255–258 (2012).
Öst, E., Joelsson, M. Ö., Burgos, C. M. & Frenckner, B. Self-assessed physical health among children with congenital diaphragmatic hernia. Pediatr. Surg. Int. 32, 493–503 (2016).
Arena, F. et al. Mid- and long-term effects on pulmonary perfusion, anatomy and diaphragmatic motility in survivors of congenital diaphragmatic hernia. Pediatr. Surg. Int. 21, 954–959 (2005).
Masumoto, K. et al. Risk of respiratory syncytial virus in survivors with severe congenital diaphragmatic hernia. Pediatr. Int. 50, 459–463 (2008).
Panitch, H. B. et al. Lung function over the first 3 years of life in children with congenital diaphragmatic hernia. Pediatr. Pulmonol. 50, 896–907 (2015).
Spoel, M. et al. Prospective longitudinal evaluation of lung function during the first year of life after repair of congenital diaphragmatic hernia. Pediatr. Crit. Care. Med. 13, e133–e139 (2012).
Prendergast, M. et al. Lung function at follow-up of infants with surgically correctable anomalies. Pediatr. Pulmonol. 47, 973–978 (2012).
Roehr, C. C. et al. Impaired somatic growth and delayed lung development in infants with congenital diaphragmatic hernia-evidence from a 10-year, single center prospective follow-up study. J. Pediatr. Surg. 44, 1309–1314 (2009).
Rygl, M. et al. Abnormalities in pulmonary function in infants with high-risk congenital diaphragmatic hernia. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech. Repub. 159, 497–502 (2015).
Muratore, C. S. et al. Pulmonary morbidity in 100 survivors of congenital diaphragmatic hernia monitored in a multidisciplinary clinic. J. Pediatr. Surg. 36, 133–140 (2001).
Kamata, S. et al. Long-term follow-up of patients with high-risk congenital diaphragmatic hernia. J. Pediatr. Surg. 40, 1833–1838 (2005).
Peetsold, M. G. et al. Pulmonary function and exercise capacity in survivors of congenital diaphragmatic hernia. Eur. Respir. J. 34, 1140–1147 (2009).
Stefanutti, G. et al. Cardiopulmonary anatomy and function in long-term survivors of mild to moderate congenital diaphragmatic hernia. J. Pediatr. Surg. 39, 526–531 (2004).
Majaesic, C. M. et al. Clinical correlations and pulmonary function at 8 years of age after severe neonatal respiratory failure. Pediatr. Pulmonol. 42, 829–837 (2007).
Haliburton, B. et al. Pulmonary function and nutritional morbidity in children and adolescents with congenital diaphragmatic hernia. J. Pediatr. Surg. 52, 252–256 (2017).
Peetsold, M. G., Vonk-Noordegraaf, A., Heij, H. H. & RJBJ, Gemke Pulmonary function and exercise testing in adult survivors of congenital diaphragmatic hernia. Pediatr. Pulmonol. 42, 325–331 (2007).
Spoel, M. et al. Pulmonary ventilation and micro-structural findings in congenital diaphragmatic hernia. Pediatr. Pulmonol. 51, 517–524 (2016).
Laviola, M. et al. Thoraco-abdominal asymmetry and asynchrony in congenital diaphragmatic hernia. Pediatr. Pulmonol. 50, 915–924 (2015).
Spoel, M. et al. Lung function in young adults with congenital diaphragmatic hernia: a longitudinal evaluation. Pediatr. Pulmonol. 48, 130–137 (2013).
Gischler, S. J. et al. A prospective comparative evaluation of persistent respiratory morbidity in esophageal atresia and congenital diaphragmatic hernia survivors. J. Pediatr. Surg. 44, 1683–1690 (2009).
Bojanić, K. et al. Cardiopulmonary exercise performance is reduced in congenital diaphragmatic hernia survivors. Pediatr. Pulmonol. 51, 1320–1329 (2016).
Trachsel, D. et al. Resting and exercise cardiorespiratory function in survivors of congenital diaphragmatic hernia. Pediatr. Pulmonol. 41, 522–529 (2006).
Van der Cammen-van Zijp, M. H. M. et al. Exercise capacity, daily activity, and severity of fatigue in term born young adults after neonatal respiratory failure. Scand. J. Med. Sci. Sports 24, 144–151 (2014).
Turchetta, A. et al. Physical activity, fitness, and dyspnea perception in children with congenital diaphragmatic hernia. Pediatr. Pulmonol. 46, 1000–1006 (2011).
Okuyama, H. et al. Correlation between lung scintigraphy and long-term outcome in survivors of congenital diaphragmatic hernia. Pediatr. Pulmonol. 41, 882–886 (2006).
Hayward, M. J. et al. Predicting inadequate long-term lung development in children with congenital diaphragmatic hernia: an analysis of longitudinal changes in ventilation and perfusion. J. Pediatr. Surg. 42, 112–116 (2007).
Lusk, L. A., Wai, K. C., Moon-Grady, A. J., Steurer, M. A. & Keller, R. L. Persistence of pulmonary hypertension by echocardiography predicts short-term outcomes in congenital diaphragmatic hernia. J. Pediatr. 166, 251–256 (2015).
Zussman, M. E., Bagby, M., Benson, D. W., Gupta, R. & Hirsch, R. Pulmonary vascular resistance in repaired congenital diaphragmatic hernia vs. age-matched controls. Pediatr. Res. 71, 697–700 (2012).
Egan, M. J. et al. Mid-term differences in right ventricular function in patients with congenital diaphragmatic hernia compared with controls. World J. Pediatr. 8, 350–354 (2012).
Iocono, J. A., Cilley, R. E., Mauger, D. T., Krummel, T. M. & Dillon, P. W. Postnatal pulmonary hypertension after repair of congenital diaphragmatic hernia: predicting risk and outcome. J. Pediatr. Surg. 34, 349–353 (1999).
Galie, N. et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 37, 67–119 (2016).
Dillon, P. W., Cilley, R. E., Mauger, D., Zachary, C. & Meier, A. The relationship of pulmonary artery pressure and survival in congenital diaphragmatic hernia. J. Pediatr. Surg. 39, 307–312 (2004).
Behrsin, J., Cheung, M. & Patel, N. Sildenafil weaning after discharge in infants with congenital diaphragmatic hernia. Pediatr. Cardiol. 34, 1844–1847 (2013).
Kinsella, J. P., Parker, T. A., Ivy, D. D. & Abman, S. H. Noninvasive delivery of inhaled nitric oxide therapy for late pulmonary hypertension in newborn infants with congenital diaphragmatic hernia. J. Pediatr. 142, 397–401 (2003).
Gischler, S. J. et al. Interdisciplinary structural follow-up of surgical newborns: a prospective evaluation. J. Pediatr. Surg. 44, 1382–1389 (2009).
Danzer, E. et al. Neurodevelopmental outcome of infants with congenital diaphragmatic hernia prospectively enrolled in an interdisciplinary follow-up program. J. Pediatr. Surg. 45, 1759–1766 (2010).
Danzer, E. et al. Longitudinal neurodevelopmental and neuromotor outcome in congenital diaphragmatic hernia patients in the first 3 years of life. J. Perinatol. 33, 893–898 (2013).
Wynn, J. et al. Developmental outcomes of children with congenital diaphragmatic hernia: a multicenter prospective study. J. Pediatr. Surg. 48, 1995–2004 (2013).
Leeuwen, L., Walker, K., Halliday, R. & Fitzgerald, D. A. Neurodevelopmental outcome in congenital diaphragmatic hernia survivors during the first three years. Early Hum. Dev. 90, 413–415 (2014).
Bevilacqua, F. et al. Neurodevelopmental outcome in congenital diaphragmatic hernia survivors: role of ventilatory time. J. Pediatr. Surg. 50, 394–398 (2015).
Danzer, E. et al. Preschool neurological assessment in congenital diaphragmatic hernia survivors: outcome and perinatal factors associated with neurodevelopmental impairment. Early Hum. Dev. 89, 393–400 (2013).
Danzer, E. et al. Neurodevelopmental outcome at one year of age in congenital diaphragmatic hernia infants not treated with extracorporeal membrane oxygenation. J. Pediatr. Surg. 50, 898–903 (2015).
Benjamin, J. R. et al. Perinatal factors associated with poor neurocognitive outcome in early school age congenital diaphragmatic hernia survivors. J. Pediatr. Surg. 48, 730–737 (2013).
Jakobson, L. S., Frisk, V., Trachsel, D. & O’Brien, K. Visual and fine-motor outcomes in adolescent survivors of high-risk congenital diaphragmatic hernia who did not receive extracorporeal membrane oxygenation. J. Perinatol. 29, 630–636 (2009).
Peetsold, M. G. et al. Psychological outcome and quality of life in children born with congenital diaphragmatic hernia. Arch. Dis. Child. 94, 834–840 (2009).
Nijhuis-van der Sanden, M. W. G. et al. Motor performance in five-year-old extracorporeal membrane oxygenation survivors: a population-based study. Crit. Care. 13, R47 (2009).
Tureczek, I. et al. Long-term motor and cognitive outcome in children with congenital diaphragmatic hernia. Acta Paediatr. Int J. Paediatr. 101, 507–512 (2012).
Madderom, M. J. et al. Neurodevelopmental, educational and behavioral outcome at 8 years after neonatal ECMO: A nationwide multicenter study. Intensive Care Med. 39, 1584–1593 (2013).
Madderom, M. J. et al. Congenital diaphragmatic hernia with (out) ECMO: impaired development at 8 years. Arch. Dis. Child Fetal Neonat. 98, F316–F322 (2013).
Kubota, A. et al. Psychosocial and cognitive consequences of major neonatal surgery. J. Pediatr. Surg. 46, 2250–2253 (2011).
Michel, F. et al. Health-related quality of life and its determinants in children with a congenital diaphragmatic hernia. Orphanet. J. Rare. Dis. 8, 89 (2013).
Toussaint, L. C. C. et al. Perceived motor competence differs from actual performance in 8-year-old neonatal ECMO survivors. Pediatrics 137, e20152724 (2016).
Bevilacqua, F. et al. Factors affecting short-term neurodevelopmental outcome in children operated on for major congenital anomalies. J. Pediatr. Surg. 50, 1125–1129 (2015).
van der Cammen-van Zijp, M. H. M. et al. Motor-function and exercise capacity in children with major anatomical congenital anomalies: an evaluation at 5 years of age. Early Hum. Dev. 86, 523–528 (2010).
Van Der Cammen-van Zijp, M. H. M. et al. Motor performance after neonatal extracorporeal membrane oxygenation: a longitudinal evaluation. Pediatrics 134, e427–e435 (2014).
1995 Joint Committee on Infant Hearing. Position Statement. American Academy of Pediatrics Joint Committee on Infant Hearing. Pediatrics 95, 152–156 (1994).
1994 Summary of the NIH consensus statement on early identification of hearing impairment in infants and young children. Md. Med. J. 43,171–172 (1994).
Jaillard, S. M. et al. Outcome at 2 years of infants with congenital diaphragmatic hernia: a population-based study. Ann. Thorac. Surg. 75, 250–256 (2003).
Robertson, C. M. T. et al. Late-onset, progressive sensorineural hearing loss after severe neonatal respiratory failure. Otol. Neurotol. 23, 353–356 (2002).
Amoils, M., Janik, M. C. & Lustig, L. R. Patterns and predictors of sensorineural hearing loss in children with congenital diaphragmatic hernia. JAMA Otolaryngol. Head. Neck Surg. 141, 923–926 (2015).
Fligor, B. J., Neault, M. W., Mullen, C. H., Feldman, H. A. & Jones, D. T. Factors associated with sensorineural hearing loss among survivors of extracorporeal membrane oxygenation therapy. Pediatrics 115, 1519–1528 (2005).
Van Den Hondel, D. et al. Sensorineural hearing loss and language development following neonatal extracorporeal membrane oxygenation. Pediatr. Crit. Care. Med. 14, 62–69 (2013).
Cortes, R. A. et al. Survival of severe congenital diaphragmatic hernia has morbid consequences. J. Pediatr. Surg. 40, 36–46 (2005).
Masumoto, K., Nagata, K., Uesugi, T., Yamada, T. & Taguchi, T. Risk factors for sensorineural hearing loss in survivors with severe congenital diaphragmatic hernia. Eur. J. Pediatr. 166, 607–612 (2007).
Javidnia, H. & Vaccani, J. P. Progressive sensorineural hearing loss in children with congenital diaphragmatic hernias. J. Otolaryngol. Head. Neck Surg. 38, 29–31 (2009).
Morando, C. et al. Hearing assessment in high-risk congenital diaphragmatic hernia survivors. Int. J. Pediatr. Otorhinolaryngol. 74, 1176–1179 (2010).
Wilson, M. G., Riley, P., Hurteau, A. M., Baird, R. & Puligandla, P. S. Hearing loss in congenital diaphragmatic hernia (CDH) survivors: is it as prevalent as we think? J. Pediatr. Surg. 48, 942–945 (2013).
Rasheed, A., Tindall, S., Cueny, D. L., Klein, M. D. & Delaney-Black, V. Neurodevelopmental outcome after congenital diaphragmatic hernia: extracorporeal membrane oxygenation before and after surgery. J. Pediatr. Surg. 36, 539–544 (2001).
Partridge, E. A. et al. Incidence and factors associated with sensorineural and conductive hearing loss among survivors of congenital diaphragmatic hernia. J. Pediatr. Surg. 49, 890–894 (2014).
Morini, F. et al. Hearing impairment in congenital diaphragmatic hernia: the inaudible and noiseless foot of time. J. Pediatr. Surg. 43, 380–384 (2008).
Dennett, K. V. et al. Sensorineural hearing loss in congenital diaphragmatic hernia survivors is associated with postnatal management and not defect size. J. Pediatr. Surg. 49, 895–899 (2014).
Bagolan, P. & Morini, F. Long-term follow up of infants with congenital diaphragmatic hernia. Semin. Pediatr. Surg. 16, 134–144 (2007).
Valfrè, L. et al. Long term follow-up in high-risk congenital diaphragmatic hernia survivors: patching the diaphragm affects the outcome. J. Pediatr. Surg. 46, 52–55 (2011).
Leeuwen, L. et al. Congenital diaphragmatic hernia and growth to 12 years. Pediatrics 140, e20163659 (2017).
Gien, J. et al. Short-term weight gain velocity in infants with congenital diaphragmatic hernia (CDH). Early Hum. Dev. 106-107, 7–12 (2017).
Pierog, A. et al. Predictors of low weight and tube feedings in children with congenital diaphragmatic hernia at 1 year of age. J. Pediatr. Gastroenterol. Nutr. 59, 527–530 (2014).
Terui, K. et al. Growth assessment and the risk of growth retardation in congenital diaphragmatic hernia: a long-term follow-up study from the Japanese Congenital Diaphragmatic Hernia Study Group. Eur. J. Pediatr. Surg. 26, 60–66 (2015).
Bairdain, S. et al. Nutritional outcomes in survivors of congenital diaphragmatic hernia (CDH)-factors associated with growth at one year. J. Pediatr. Surg. 50, 74–77 (2015).
Haliburton, B. et al. Long-term nutritional morbidity for congenital diaphragmatic hernia survivors: failure to thrive extends well into childhood and adolescence. J. Pediatr. Surg. 50, 734–738 (2015).
Muratore, C. S., Utter, S., Jaksic, T., Lund, D. P. & Wilson, J. M. Nutritional morbidity in survivors of congenital diaphragmatic hernia. J. Pediatr. Surg. 36, 1171–1176 (2001).
Koziarkiewicz, M., Taczalska, A. & Piaseczna-Piotrowska, A. Long-term follow-up of children with congenital diaphragmatic hernia--observations from a single institution. Eur. J. Pediatr. Surg. 24, 500–507 (2014).
Haliburton, B. et al. Nutritional intake, energy expenditure, and growth of infants following congenital diaphragmatic hernia repair. J. Pediatr. Gastroenterol. Nutr. 62, 474–478 (2016).
Caruso, A. M. et al. Gastroesophageal reflux in patients treated for congenital diaphragmatic hernia: short- and long-term evaluation with multichannel intraluminal impedance. Pediatr. Surg. Int. 29, 553–559 (2013).
Arena, F. et al. Gastrointestinal sequelae in survivors of congenital diaphragmatic hernia. Pediatr. Int. 50, 76–80 (2008).
Peetsold, M. G. et al. Congenital diaphragmatic hernia: long-term risk of gastroesophageal reflux disease. J. Pediatr. Gastroenterol. Nutr. 51, 448–453 (2010).
Terui, K. et al. Prognostic factors of gastroesophageal reflux disease in congenital diaphragmatic hernia: a multicenter study. Pediatr. Surg. Int. 30, 1129–1134 (2014).
Chamond, C., Morineau, M., Gouizi, G., Bargy, F. & Beaudoin, S. Preventive antireflux surgery in patients with congenital diaphragmatic hernia. World J. Surg. 32, 2454–2458 (2008).
Vandenplas, Y. et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J. Pediatr. Gastroenterol. Nutr. 49, 498–547 (2009).
Maier, S. et al. Preventive antireflux surgery in neonates with congenital diaphragmatic hernia: a single-blinded prospective study. J. Pediatr. Surg. 46, 1510–1515 (2011).
Dariel, A., Roze, J. C., Piloquet, H., Podevin, G. & CDHSG, French Impact of prophylactic fundoplication on survival without growth disorder in left congenital diaphragmatic hernia requiring a patch repair. J. Pediatr. 157, 688–690 (2010). 690e681.
Steven, M. J., Fyfe, A. H. B., Raine, P. A. M. & Watt, I. Esophageal adenocarcinoma: a long-term complication of congenital diaphragmatic hernia? J. Pediatr. Surg. 42, e1–e3 (2007).
Lally, K. P. et al. Standardized reporting for congenital diaphragmatic hernia—an international consensus. J. Pediatr. Surg. 48, 2408–2415 (2013).
St. Peter, S. D. et al. Abdominal complications related to type of repair for congenital diaphragmatic hernia. J. Surg. Res. 140, 234–236 (2007).
Jancelewicz, T., Chiang, M., Oliveira, C. & Chiu, P. P. Late surgical outcomes among congenital diaphragmatic hernia (CDH) patients: Why long-term follow-up with surgeons is recommended. J. Pediatr. Surg. 48, 935–941 (2013).
Jancelewicz, T. et al. Long-term surgical outcomes in congenital diaphragmatic hernia: observations from a single institution. J. Pediatr. Surg. 45, 155–160 (2010).
Tsai, J., Sulkowski, J., Adzick, N. S., Hedrick, H. L. & Flake, A. W. Patch repair for congenital diaphragmatic hernia: is it really a problem? J. Pediatr. Surg. 47, 637–641 (2012).
Nasr, A., Struijs, M. C., Ein, S. H., Langer, J. C. & Chiu, P. P. L. Outcomes after muscle flap vs prosthetic patch repair for large congenital diaphragmatic hernias. J. Pediatr. Surg. 45, 151–154 (2010).
Laituri, C. A., Garey, C. L., Ostlie, D. J., Holcomb, G. W. & St. Peter, S. D. Morgagni hernia repair in children: comparison of laparoscopic and open results. J. Laparoendosc. Adv. Surg. Techn 21, 89–91 (2011).
Nagata, K. et al. Risk factors for the recurrence of the congenital diaphragmatic hernia-report from the long-term follow-up study of Japanese CDH study group. Eur. J. Pediatr. Surg. 25, 9–14 (2015).
Wessel, L. M., Fuchs, J. & Rolle, U. The surgical correction of congenital deformities: the treatment of diaphragmatic hernia, esophageal atresia and small bowel atresia review. Dtsch. Arztebl. Int. 112, 357–364 (2015).
Cho, S. D. et al. Analysis of 29 consecutive thoracoscopic repairs of congenital diaphragmatic hernia in neonates compared to historical controls. J. Pediatr. Surg. 44, 80–86 (2009).
Putnam, L. R. et al. Factors associated with early recurrence after congenital diaphragmatic hernia repair. J. Pediatr. Surg. 52, 928–932 (2017).
Ward, E. P. et al. Preemptive Ladd in congenital diaphragmatic hernia and Abdominal Wall defects does not reduce the risk of future volvulus. J. Pediatr. Surg. 52, 1956–1961 (2017).
Yawn, B. P. et al. A population-based study of school scoliosis screening. JAMA 282, 1427–1432 (1999).
Kuklová, P. et al. Large diaphragmatic defect: are skeletal deformities preventable? Pediatr. Surg. Int. 27, 1343–1349 (2011).
Russell, K. W., Barnhart, D. C., Rollins, M. D., Hedlund, G. & Scaife, E. R. Musculoskeletal deformities following repair of large congenital diaphragmatic hernias. J. Pediatr. Surg. 49, 886–889 (2014).
Institute for Relevant Clinical Data Analytics (www.scamps.org).
Farias, M. et al. Standardized Clinical Assessment And Management Plans (SCAMPs) provide a better alternative to clinical practice guidelines. Health Aff. 32, 911–920 (2013).
Acknowledgements
Wichor Bramer, biomedical information specialist of the Medical Library of the Erasmus MC in Rotterdam, assisted with the extensive literature research.
Members of the CDH EURO Consortium Group
B. Urlesberger, H. Till, A. Debeer, J. Deprest, R. Keijzer, A. Benachi, M. Mokhtari, L. Storme, P. Tissieres, F. Kipfmueller, T. Schaible, L. Wessel, C. Breatnach, N. Patel, C. Davis, E. Leva, F. Ciralli, P. Bagolan, I. Capolupo, A. Braguglia, F. Morini, R. Emblem, K. Ertesvag, I. Maroszynska, B. Frenckner, C. Mesas, D. Elorza, L. Martinez, A.F.J. van Heijst, I. de Blaauw, H. Scharbatke, H. IJsselstijn, U.S. Kraemer, D. Tibboel, R.M.H. Wijnen, J. Deprest, P. De Coppi, S. Eaton, A. Hoskote, M. Davenport, and A. Greenough
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
IJsselstijn, H., Breatnach, C., Hoskote, A. et al. Defining outcomes following congenital diaphragmatic hernia using standardised clinical assessment and management plan (SCAMP) methodology within the CDH EURO consortium. Pediatr Res 84, 181–189 (2018). https://doi.org/10.1038/s41390-018-0063-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-018-0063-3
This article is cited by
-
Functional lung imaging of 2-year-old children after congenital diaphragmatic hernia repair using dynamic mode decomposition MRI
European Radiology (2023)
-
Long-term pulmonary outcome of children with congenital diaphragmatic hernia: functional lung MRI using matrix-pencil decomposition enables side-specific assessment of lung function
European Radiology (2023)
-
Congenital diaphragmatic hernia
Nature Reviews Disease Primers (2022)
-
MR lung perfusion measurements in adolescents after congenital diaphragmatic hernia: correlation with spirometric lung function tests
European Radiology (2022)
-
A systematic review and meta-analysis of surgical morbidity of primary versus patch repaired congenital diaphragmatic hernia patients
Scientific Reports (2021)