Abstract
Background
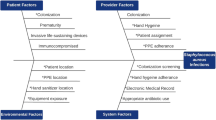
Staphylococcus aureus is the leading cause of skin and soft tissue infections (SSTIs). To develop interventions to prevent recurrent infections, household attributes and individual practices influencing S. aureus colonization must be discerned.
Methods
Households of healthy children with methicillin-resistant S. aureus (MRSA) SSTI (n = 150; 671 participants) were interviewed regarding health history, activities, and hygiene practices. S. aureus colonization was assessed in household members, and recovered isolates were typed by repetitive sequence-based PCR.
Results
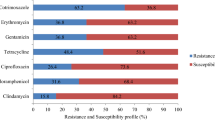
The number of unique strain types in a household (median 1, range 0–7) correlated with the number of colonized individuals (p < 0.001). The MRSA infecting strain type colonized a household member in 57% of 91 households with an available infecting strain, and was the most common strain type recovered in 45% of these households. In multivariable models, household MRSA colonization burden (p < 0.001), sharing a bedroom with MRSA-colonized individuals (p = 0.03), renting dwelling (p = 0.048), and warmer seasons (p = 0.02) were associated with increased MRSA colonization. Increasing age (p = 0.02), bathing at least daily (p = 0.01), and antibacterial soap use (p = 0.03) correlated with reduced MRSA colonization.
Conclusions
This study identified practices that correlate with MRSA colonization, which will inform physician counseling and multifaceted interventions among MRSA-affected households to mitigate MRSA in the community.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Dukic, V. M., Lauderdale, D. S., Wilder, J., Daum, R. S. & David, M. Z. Epidemics of community-associated methicillin-resistant Staphylococcus aureus in the United States: a meta-analysis. PloS ONE 8, e52722 (2013).
Moran, G. J. et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355, 666–674 (2006).
Otto, M. Community-associated MRSA: a dangerous epidemic. Future Microbiol. 2, 457–459 (2007).
Knox, J., Uhlemann, A.-C. & Lowy, F. D. Staphylococcus aureus infections: transmission within households and the community. Trends Microbiol. 23, 437–444 (2015).
Alam, M. T. et al. Transmission and microevolution of USA300 MRSA in U.S. households: evidence from whole-genome sequencing. mBio 6, e00054–15 (2015).
Fritz, S. A. et al. Household versus individual approaches to eradication of community-associated Staphylococcus aureus in children: a randomized trial. Clin. Infect. Dis. 54, 743–751 (2012).
Diep, B. A. et al. Emergence of multidrug-resistant, community-associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with men. Ann. Intern Med. 148, 249–257 (2008).
Wertheim, H. F. L. et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 5, 751–762 (2005).
Fritz, S. A., Epplin, E. K., Garbutt, J. & Storch, G. A. Skin infection in children colonized with community-associated methicillin-resistant Staphylococcus aureus. J. Infect. 59, 394–401 (2009).
Nouwen, J. L. et al. Predicting the Staphylococcus aureus nasal carrier state: derivation and validation of a “culture rule." Clin. Infect. Dis. 39, 806–811 (2004).
Costello, E. K. et al. Bacterial community variation in human body habitats across space and time. Science 326, 1694–1697 (2009).
Kluytmans, J., van Belkum, A. & Verbrugh, H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol Rev. 10, 505–520 (1997).
Knox, J. et al. Environmental contamination as a risk factor for intra-household Staphylococcus aureus transmission. PloS ONE 7, e49900 (2012).
Uhlemann, A.-C. et al. The environment as an unrecognized reservoir for community-associated methicillin resistant Staphylococcus aureus USA300: a case-control study. PloS ONE 6, e22407 (2011).
Fritz, S. A. et al. Staphylococcus aureus colonization in children with community-associated Staphylococcus aureus skin infections and their household contacts. Arch. Pediatr. Adolesc. Med. 166, 551–557 (2012).
Miller, L. G. et al. Staphylococcus aureus colonization among household contacts of patients with skin infections: risk factors, strain discordance, and complex ecology. Clin. Infect. Dis. 54, 1523–1535 (2012).
Liu, C. et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin. Infect. Dis. 52, 285–292 (2011).
Kaplan, S. L. et al. Randomized trial of “bleach baths” plus routine hygienic measures vs. routine hygienic measures alone for prevention of recurrent infections. Clin. Infect. Dis. 58, 679–682 (2014).
Fritz, S. A., Garbutt, J., Elward, A., Shannon, W. & Storch, G. A. Prevalence of and risk factors for community-acquired methicillin-resistant and methicillin-sensitive Staphylococcus aureus colonization in children seen in a practice-based research network. Pediatrics 121, 1090–1098 (2008).
Rodriguez, M., Hogan, P. G., Krauss, M., Warren, D. K. & Fritz, S. A. Measurement and impact of Staphylococcus aureus colonization pressure in households. J. Pediatr. Infect. Dis. Soc. 2, 147–154 (2013).
Halliday, G. & Snowdon, J. The Environmental Cleanliness and Clutter Scale (ECCS). Int Psychogeriatr. 21, 1041–1050 (2009).
Fritz, S. A. et al. Contamination of environmental surfaces with Staphylococcus aureus in households with children infected with methicillin-resistant S aureus. JAMA Pediatr. 168, 1030–1038 (2014).
Cockerill, F. R. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-third Informational Supplement; M100 - S23 (CLSI, Wayne, PA, 2013).
Boye, K., Bartels, M. D., Andersen, I. S., Møller, J. A. & Westh, H. A new multiplex PCR for easy screening of methicillin-resistant Staphylococcus aureus SCCmec types I-V. Clin. Microbiol Infect. 13, 725–727 (2007).
Rodriguez, M. et al. Discriminatory indices of typing methods for epidemiologic analysis of contemporary Staphylococcus aureus strains. Medicine 94, e1534 (2015).
Del Vecchio, V. G. et al. Molecular genotyping of methicillin-resistant Staphylococcus aureus via fluorophore-enhanced repetitive-sequence PCR. J. Clin. Microbiol. 33, 2141–2144 (1995).
R Core Team. R: A Language and Environment for Statistical Computing. [Internet] (R Foundation for Statistical Computing, Vienna, Austria; 2016). Available at https://www.R-project.org/
Waskom, M., et al. Seaborn: V0.8.0 (Zenodo, 2017).
Wang, K., Gaitsch, H., Poon, H., Cox, N. J. & Rzhetsky, A. Classification of common human diseases derived from shared genetic and environmental determinants. Nat. Genet. 49, 1319–1325 (2017).
Arguez, A. et al. NOAA’s 1981–2010 U.S. climate normals: an overview. Bull. Am. Meteorol. Soc. 93, 1687–1697 (2012).
Blanco, N. et al. Effect of meteorological factors and geographic location on methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci colonization in the US. PloS One 12, e0178254 (2017).
Wang, X., Towers, S., Panchanathan, S. & Chowell, G. A population based study of seasonality of skin and soft tissue infections: implications for the spread of CA-MRSA. PloS ONE 8, e60872 (2013).
Sahoo, K. C. et al. Climatic factors and community-associated methicillin-resistant Staphylococcus aureus skin and soft-tissue infections - a time-series analysis study. Int J. Environ. Res Public Health 11, 8996–9007 (2014).
Knox, J. et al. Community-associated methicillin-resistant Staphylococcus aureus transmission in households of infected cases: a pooled analysis of primary data from three studies across international settings. Epidemiol. Infect. 143, 354–365 (2015).
Food and Drug Administration. Fischer, A. Press Announcements - FDA issues final rule on safety and effectiveness of antibacterial soaps (2016) https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm517478.htm.
Nerby, J. M. et al. Risk factors for household transmission of community-associated methicillin-resistant Staphylococcus aureus. Pediatr. Infect. Dis. J. 30, 927–932 (2011).
Honisch, M., Stamminger, R. & Bockmühl, D. P. Impact of wash cycle time, temperature and detergent formulation on the hygiene effectiveness of domestic laundering. J. Appl. Microbiol. 117, 1787–1797 (2014).
Acknowledgements
We thank Meghan Wallace and Angela Shupe for their assistance with molecular strain typing. For their assistance in patient referral, we acknowledge Mary Bixby, RN, Cardinal Glennon Children’s Hospital; Rachel Orscheln, MD, Washington University; Jennifer Seigel, RN, PNP, and the St. Louis Children’s Hospital Pediatric Ambulatory Wound Service staff; and Jane Garbutt, MB, ChB, Sherry Dodd, and the physicians and staff of participating Washington University Pediatric and Adolescent Ambulatory Research Consortium practices, including Mercy Clinic Pediatrics – Union and Washington, Johnson Pediatric Center, Heartland Pediatrics, Forest Park Pediatrics, Tots Thru Teens, Pediatric Healthcare Unlimited, Northwest Pediatrics, Esse Health Pediatric & Adolescent Medicine – Watson Road, Fenton Pediatrics, Blue Fish Pediatrics, and Southwest Pediatrics. We appreciate the thoughtful review of this manuscript by David Hunstad, MD. This work was supported by the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital [to S.A.F.]; National Institutes of Health/National Institute of Allergy and Infectious Diseases [grant number K23-AI091690 to S.A.F.]; the National Center for Advancing Translational Sciences at the National Institutes of Health [grant number UL1-TR002345 to S.A.F.]; the Agency for Healthcare Research and Quality [grant numbers R01-HS021736, R01-HS024269 to S.A.F.]; and the Burroughs Wellcome Foundation Investigators in the Pathogenesis of Infectious Disease Award [to J.B.W.]. The computational analysis was partially funded by DARPA Big Mechanism program under ARO contract W911NF1410333 [to A.R.]; by NIH grants R01HL122712, 1P50MH094267, U01HL108634 [to A.R.]; and a gift from Liz and Kent Dauten [to A.R.]. These funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Mork, R.L., Hogan, P.G., Muenks, C.E. et al. Comprehensive modeling reveals proximity, seasonality, and hygiene practices as key determinants of MRSA colonization in exposed households. Pediatr Res 84, 668–676 (2018). https://doi.org/10.1038/s41390-018-0113-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-018-0113-x
This article is cited by
-
Transmission routes of antibiotic resistant bacteria: a systematic review
BMC Infectious Diseases (2022)
-
Effect of climate on surgical site infections and anticipated increases in the United States
Scientific Reports (2022)
-
Early development of the skin microbiome: therapeutic opportunities
Pediatric Research (2021)
-
Changing epidemiology of methicillin-resistant Staphylococcus aureus in a low endemicity area—new challenges for MRSA control
European Journal of Clinical Microbiology & Infectious Diseases (2020)
-
Prevention Strategies for Recurrent Community-Associated Staphylococcus aureus Skin and Soft Tissue Infections
Current Infectious Disease Reports (2019)