Abstract
Background
Left ventricular noncompaction (LVNC) is a primary cardiomyopathy with heterogeneous genetic origins. The aim of this study was to elucidate the role of sarcomere gene variants in the pathogenesis and prognosis of LVNC.
Methods and results
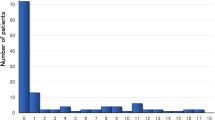
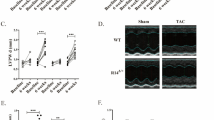
We screened 82 Japanese patients (0–35 years old), with a diagnosis of LVNC, for mutations in seven genes encoding sarcomere proteins, by direct DNA sequencing. We identified variants in a significant proportion of cases (27%), which were associated with poor prognosis (p = 0.012), particularly variants in TPM1, TNNC1, and ACTC1 (p = 0.012). To elucidate the pathological role, we developed and studied human-induced pluripotent stem cells (hiPSCs) from a patient carrying a TPM1 p.Arg178His mutation, who underwent heart transplantation. These cells displayed pathological changes, with mislocalization of tropomyosin 1, causing disruption of the sarcomere structure in cardiomyocytes, and impaired calcium handling. Microarray analysis indicated that the TPM1 mutation resulted in the down-regulation of the expression of numerous genes involved in heart development, and positive regulation of cellular process, especially the calcium signaling pathway.
Conclusions
Sarcomere genes are implicated as genetic triggers in the development of LVNC, regulating the expression of numerous genes involved in heart development, or modifying the severity of disease.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Chin, T. K., Perloff, J. K., Williams, R. G., Jue, K. & Mohrmann, R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 82, 507–513 (1990).
Maron, B. J. et al. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation 113, 1807–1816 (2006).
Ichida, F. et al. Novel gene mutations in patients with left ventricular noncompaction or barth syndrome. Circulation 103, 1256–1263 (2001).
Klaassen, S. et al. Mutations in sarcomere protein genes in left ventricular noncompaction. Circulation 117, 2893–2901 (2008).
Hoedemaekers, Y. M. et al. The importance of genetic counseling, DNA diagnostics, and cardiologic family screening in left ventricular noncompaction cardiomyopathy. Circ. Cardiovasc. Genet. 3, 232–239 (2010).
Wang, C. et al. A wide and specific spectrum of genetic variants and genotype–phenotype correlations revealed by next-generation sequencing in patients with left ventricular noncompaction. J. Am. Heart Assoc. 6, e006210 (2017).
Nomura, Y. et al. A novel MYH7 gene mutation in a fetus with left ventricular noncompaction. Can. J. Cardiol. 31, 103.e1-3 (2015).
Sedmera, D., Pexieder, T., Vuillemin, M., Thompson, R. P. & Anderson, R. H. Developmental pattern of the myocardium. Anat. Rec. 28, 319–337 (2000).
Jefferies, J. L. et al. Cardiomyopathy phenotypes and outcomes for children with left ventricular myocardial noncompaction: Results from the pediatric cardiomyopathy registry. J. Card. Fail. 21, 877–884 (2015).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the american college of medical genetics and genomics and the association for molecular pathology. Genet. Med. 17, 405–424 (2015).
Ojala, M. et al. Culture conditions affect cardiac differentiation potential of human pluripotent stem cells. PLoS ONE 7, e48659 (2012).
Uosaki, H. et al. Efficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expression. PLoS ONE 6, e23657 (2011).
RJ, Parks & Howlett, S. E. H-89 decreases the gain of excitation-contraction coupling and attenuates calcium sparks in the absence of beta-adrenergic stimulation. Eur. J. Pharmacol. 691, 163–172 (2012).
Mercado, J. et al. Local control of TRPV4 channels by AKAP 150-targeted PKC in arterial smooth muscle. J. Gen. Physiol. 143, 559–575 (2014).
Nihei, K. et al. Wolff–Parkinson–White (WPW) syndrome in isolated noncompaction of the ventricular myocardium (INVM). Circ. J. 68, 82–84 (2004).
Chang, B. et al. Identification of a novel TPM1 mutation in a family with left ventricular noncompaction and sudden death. Mol. Genet. Metab. 102, 200–206 (2011).
Yoshida, Y. et al. A novel ACTC1 gene mutation in a young boy with left ventricular noncompaction and arrhythmias. Heart Rhythm Rep. 2, 92–97 (2016).
Huang da, W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Maron, B. J., Maron, M. S. & Semsarian, C. Genetics of hypertrophic cardiomyopathy after 20 years: clinical perspectives. J. Am. Coll. Cardiol. 60, 705–715 (2012).
Otsuka, H. et al. Prevalence and distribution of sarcomeric gene mutations in Japanese patients with familial hypertrophic cardiomyopathy. Circ. J. 76, 453–461 (2012).
Tian, T. et al. A low prevalence of sarcomeric gene variants in a chinese cohort with left ventricular non-compaction. Heart Vessels 30, 258–264 (2015).
Miszalski-Jamka, K. et al. Novel genetic triggers and genotype–phenotype correlations in patients with left ventricular noncompaction. Circ. Cardiovasc Genet. 10, e001763 (2017).
Landstrom, A. P. et al. Molecular and functional characterization of novel hypertrophic cardiomyopathy susceptibility mutations in TNNC1-encoded troponin c. J. Mol. Cell. Cardiol. 45, 281–288 (2008).
Parvatiyar, M. S. et al. A mutation in TNNC1-encoded cardiac troponin c, TNNC1-a31s, predisposes to hypertrophic cardiomyopathy and ventricular fibrillation. J. Biol. Chem. 287, 31845–33155 (2012).
Chung, W. K., Kitner, C. & Maron, B. J. Novel frameshift mutation in troponin c (TNNC1) associated with hypertrophic cardiomyopathy and sudden death. Cardiol. Young 21, 345–348 (2011).
Mogensen, J. et al. Severe disease expression of cardiac troponin C and T mutations in patients with idiopathic dilated cardiomyopathy. J. Am. Coll. Cardiol. 44, 2033–2040 (2004).
Kamisago, M. et al. Mutations in sarcomere protein genes as a cause of dilated cardiomyopathy. N. Engl. J. Med. 343, 1688–1696 (2000).
Moller, D. V. et al. The role of sarcomere gene mutations in patients with idiopathic dilated cardiomyopathy. Eur. J. Hum. Genet. 17, 1241–1249 (2009).
Herman, D. S. et al. Truncations of titin causing dilated cardiomyopathy. N. Engl. J. Med. 366, 619–628 (2012).
Redwood, C. & Robinson, P. Alpha-tropomyosin mutations in inherited cardiomyopathies. J. Muscle Res. Cell Motil. 34, 285–294 (2013).
Orzechowski, M., Fischer, S., Moore, J. R., Lehman, W. & Farman, G. Energy landscapes reveal the myopathic effects of tropomyosin mutations. Arch. Biochem. Biophys. 564, 89–99 (2014).
Janco, M. et al. Alpha-tropomyosin with a D175N or E180G mutation in only one chain differs from tropomyosin with mutations in both chains. Biochemistry 51, 9880–9890 (2012).
Zheng, W., Hitchcock-DeGregori, S. E. & Barua, B. Investigating the effects of tropomyosin mutations on its flexibility and interactions with filamentous actin using molecular dynamics simulation. J. Muscle Res. Cell Motil. 37, 131–147 (2016).
Loong, C. K., Zhou, H. X. & Chase, P. B. Familial hypertrophic cardiomyopathy related E180G mutation increases flexibility of human cardiac alpha-tropomyosin. FEBS Lett. 586, 3503–3507 (2012).
Loong, C. K., Badr, M. A. & Chase, P. B. Tropomyosin flexural rigidity and single ca regulatory unit dynamics: implications for cooperative regulation of cardiac muscle contraction and cardiomyocyte hypertrophy. Front. Physiol. 3, 80 (2012).
Hershberger, R. E. et al. Genetic evaluation of cardiomyopathy—a Heart Failure Society of America Practice Guideline. J. Card. Fail. 15, 83–97 (2009).
Acknowledgements
We gratefully acknowledge W, Chung, L.J. Addnizio, and all Noncompaction study collaborators (Supplement S8). We are also grateful to the patients and families for their participation and to the referring clinicians for supporting the study. We thank Professor Yuichi Adachi for the steadfast counsel and guidance. This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology in Japan (Research Project Number: 15K09685, 24591571, and 17591072) and by Japan Heart Foundation Research Grant on Dilated Cardiomyopathy to F.I.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Takasaki, A., Hirono, K., Hata, Y. et al. Sarcomere gene variants act as a genetic trigger underlying the development of left ventricular noncompaction. Pediatr Res 84, 733–742 (2018). https://doi.org/10.1038/s41390-018-0162-1
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-018-0162-1
This article is cited by
-
The Genetic Factors Influencing Cardiomyopathies and Heart Failure across the Allele Frequency Spectrum
Journal of Cardiovascular Translational Research (2024)
-
Modeling Nonischemic Genetic Cardiomyopathies Using Induced Pluripotent Stem Cells
Current Cardiology Reports (2022)
-
Overlap phenotypes of the left ventricular noncompaction and hypertrophic cardiomyopathy with complex arrhythmias and heart failure induced by the novel truncated DSC2 mutation
Orphanet Journal of Rare Diseases (2021)
-
A comprehensive guide to genetic variants and post-translational modifications of cardiac troponin C
Journal of Muscle Research and Cell Motility (2021)
-
Left Ventricular Noncompaction Syndrome: Genetic Insights and Therapeutic Perspectives
Current Cardiology Reports (2020)