Abstract
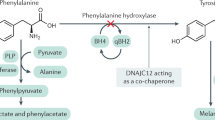
Despite a strict dietary control, patient with hyperphenylalaninemia or phenylketonuria may show cognitive and/or behavioral disorders. These comorbid deficits are of great concern to patients, families, and health organizations. However, biomarkers capable of detecting initial stages of neurological damage are not commonly employed. The pathogenesis of phenylketonuria is complex in nature. Increasingly, the role of oxidative stress has gained acceptance and biomarkers reflecting oxidative damage to the brain and easily accessible in peripheral biofluids have been validated using mass spectrometry techniques. In the present review, the role of oxidative stress in the pathogenesis of phenylketonuria and hyperphenylalaninemia has been updated. Moreover, we report on newly validated brain-specific lipid peroxidation biomarkers and inform on their relevance in the detection and monitoring of neurological damage in phenylketonuric patients. In preliminary studies, a correlation between lipid peroxidation biomarkers and neurological dysfunction in patients with PKU was reported. However, there is a need of adequately powered trials to confirm the validity of these biomarkers for early detection of brain damage, initiation of treatment, and reliably monitor evolving disease both in phenylketonuria and hyperphenylalaninemia.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Blau, N., van Spronsen, F. J. & Levy, H. L. Phenylketonuria. Lancet 376, 1417–1427 (2010).
Réblová, K., Kulhánek, P. & Fajkusová, L. Computational study of missense mutations in phenylalanine hydroxylase. J. Mol. Model. 21, 70–77 (2015).
van Wegberg, A. M. J. et al. The complete European guidelines on phenylketonuria: diagnosis and treatment. Orphanet J. Rare Dis. 12, 162–169 (2017).
Vockley, J. et al. Phenylalanine hydroxylase deficiency: diagnosis and management guideline. Genet. Med. 16, 188–200 (2014).
Schuck, P. F. et al. Phenylketonuria pathophysiology: on the role of metabolic alterations. Aging Dis. 6, 390–399 (2015).
Stepien, K. M. et al. Evidence of oxidative stress and secondary mitochondrial dysfunction in metabolic and non-metabolic disorders. J. Clin. Med. 6, 71 (2017).
Feillet, F. et al. Challenges and pitfalls in the management of phenylketonuria. Pediatrics 126, 333–341 (2010).
Blasco, H. et al. A multiplatform metabolomics approach to characterize plasma levels of phenylalanine and tyrosine in phenylketonuria. JIMD Rep. 32, 69–79 (2017).
Huttenlocher, P. R. The neuropathology of phenylketonuria: human and animal studies. Eur. J. Pediatr. 159, S102–S106 (2000).
Hood, A. et al. Prolonged exposure to high and variable phenylalanine levels over the lifetime predicts brain white matter integrity in children with phenylketonuria. Mol. Genet. Metab. 114, 19–24 (2015).
Hood, A. et al. Brain white matter integrity mediates the relationship between phenylalanine control and executive abilities in children with phenylketonuria. JIMD Rep. 33, 41–47 (2017).
Velema, M. et al. Parkinsonism in phenylketonuria: a consequence of dopamine depletion? JIMD Rep. 20, 35–38 (2015).
Kyprianou, N. et al. Assessment of mitochondrial respiratory chain function in hyperphenylalaninemia. J. Inherit. Metab. Dis. 32, 289–296 (2009).
Ribas, G. S. et al. Oxidative stress in phenylketonuria: what is the evidence? Cell Mol. Neurobiol. 31, 653–662 (2011).
Torres-Cuevas, I. et al. Oxygen and oxidative stress in the perinatal period. Redox Biol. 12, 674–681 (2017).
Jones, D. P. Redox sensing: ortogonal control in cell cycle and apoptosis. J. Inter Med. 268, 432–448 (2010).
Jones, D. P. & Sies, H. The redox code. Antioxid. Redox Signal. 23, 734–746 (2015).
Sanayama, Y. et al. Experimental evidence that phenylalanine is strongly associated to oxidative stress in adolescents and adults with phenylketonuria. Mol. Genet. Metab. 103, 220–225 (2011).
Kumru, B. et al. Effect of blood phenylalanine levels on oxidative stress in classical phenylketonuric patients. Cell Mol. Neurobiol. 38, 1033–1038 (2018).
Bodley, J. L. et al. Low iron stores in infants and children with treated phenylketonuria: a population at risk for iron-deficiency anaemia and associated cognitive deficits. Eur. J. Pediatr. 152, 140–143 (1993).
Artuch, R. et al. Plasma phenylalanine is associated with decreased serum ubiquinone-10 concentrations in phenylketonuria. J. Inherit. Metab. Dis. 24, 359–366 (2001).
Sitta, A. et al. L-carnitine blood level and oxidant stress in treated phenylketonuric patients. Cell Mol. Neurobiol. 29, 211–218 (2009).
Rech, V. C. et al. Inhibition of the mitochondrial respiratory chain by phenylalanine in rat cerebral cortex. Neurochem Res. 27, 353–357 (2002).
Preissler, T. et al. Phenylalanine induces oxidative stress and decreases the viability of rat astrocytes: possible relevance for the pathophysiology of neurodegeneration in phenylketonuria. Metab. Brain Dis. 31, 529–537 (2016).
Fernandes, C. G. et al. Experimental evidence that phenylalanine provokes oxidative stress in hippocampus and cerebral cortex of developing rats. Cell Mol. Neurobiol. 30, 317–326 (2010).
da Fonseca, R. R., Johnson, W. E., O'Brien, S. J., Vasconcelos, V. & Antunes, A. Molecular evolution and the role of oxidative stress in the expansion and functional diversification of cytosolic glutathione transferases. BMC Evol. Biol. 10, 281–291 (2010).
Moraes, T. B. et al. Glutathione metabolism enzymes in brain and liver of hyperphenylalaninemic rats and the effect of lipoic acid treatment. Metab. Brain Dis. 29, 609–615 (2014).
Moraes, T. B. et al. Role of catalase and superoxide dismutase activities on oxidative stress in the brain of a phenylketonuria animal model and the effect of lipoic acid. Cell Mol. Neurobiol. 33, 253.60 (2013).
Rocha, J. C. & Martins, M. J. Oxidative stress in phenylketonuria: future directions. J. Inherit. Metab. Dis. 35, 381–398 (2012).
Deon, M. et al. Urinary biomarkers of oxidative stress and plasmatic inflammatory profile in Phenylketonuric treated patients. Int J. Dev. Neurosci. 47, 259–265 (2015).
Ekin, S., Dogan, M., Gok, F. & Karakus, Y. Assessment of antioxidant enzymes, total sialic acid, lipid bound sialic acid, vitamins and selected amino acids in children with phenylketonuria. Pediatr. Res. https://doi.org/10.1038/s41390-018-0137-2 (2018).
van Bakel, M. M. E. et al. Antioxidant and thyroid hormone status in selenium-deficient phenylketonuric and hyperphenylalaninemic patients. Am. J. Clin. Nutr. 72, 976–981 (2000).
Sirtori, L. R. et al. Oxidative stress in patients with phenylketonuria. Biochim. Biophys. Acta 1740, 68–73 (2005).
Sitta, A. et al. Evidence that L-carnitine and selenium supplementation reduces oxidative stress in phenylketonuric patients. Cell Mol. Neurobiol. 31, 429–436 (2011).
Sitta, A. et al. Investigation of oxidative stress parameters in treated phenylketonuric patients. Metab. Brain Dis. 21, 287–296 (2006).
Wilke, B. C. et al. Selenium, glutathione peroxidase (GSH-Px) and lipid peroxidation products before and after selenium supplementation. Clin. Chim. Acta 207, 137–142 (1992).
Colomé, C. et al. Lipophilic antioxidants in patients with phenylketonuria. Am. J. Clin. Nutr. 77, 185–188 (2003).
Schulpis, K. H. et al. Effect of diet on plasma total antioxidant status in phenylketonuric patients. Eur. J. Clin. Nutr. 57, 383–387 (2003).
Schulpis, K. H. et al. Low total antioxidant status is implicated with high 8-hydroxy-2-deoxyguanosine serum concentrations in phenylketonuria. Clin. Biochem. 38, 239–242 (2005).
Sitta, A. et al. Evidence that DNA damage is associated to phenylalanine blood levels in leukocytes from phenylketonuric patients. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 679, 13–16 (2009).
Sitta, A. et al. Effect of short- and long-term exposition to high phenylalanine blood levels on oxidative damage in phenylketonuric patients. Int. J. Dev. Neurosci. 27, 243–247 (2009).
Veyrat-Durebex, C. et al. Hyperphenylalaninemia correlated with global decrease of antioxidant genes expression in white blood cells of adult patients with phenylketonuria. JIMD Rep. 37, 73–83 (2017).
Item, C. B. et al. Demethylation of the promoter region of GPX3 in a newborn with classical phenylketonuria. Clin. Biochem. 50, 159–161 (2017).
Olsen, R. K., Cornelius, N. & Gregersen, N. Redox signaling and mitochondrial stress responses; lessons from inborn errors of metabolism. J. Inherit. Metab. Dis. 38, 703–719 (2015).
Naudí, A. et al. Lipidomics of human brain aging and Alzheimer’s disease pathology. Int Rev. Neurobiol. 122, 133–189 (2015).
Sastry, P. S. Lipids of nervous tissue: composition and metabolism. Prog. Lipid Res 24, 69–176 (1985).
Jahn, U., Galano, J. M. & Durand, T. Beyond prostaglandins—chemistry and biology of cyclic oxygenated metabolites formed by free-radical pathways from polyunsaturated fatty acids. Angew. Chem. Int. Ed. Engl. 47, 5894–5955 (2008).
Van Rollins, M., Woltjer, R. L., Yin, H., Morrow, J. D. & Montine, T. J. F2-Dihomo-isoprostanes arise from free radical attack on adrenic acid. J. Lip. Res. 49, 995–1005 (2008).
García-Blanco, A. et al. Reliable determination of new lipid peroxidation compounds as potential early Alzheimer disease biomarkers. Talanta 184, 193–201 (2018).
Signorini, C., et al. Isoprostanes and 4-Hydroxy-2-nonenal: markers or mediators of disease? Focus on Rett Syndrome as a model of autism spectrum disorder. Oxid. Med. Cell. Longev. 2013, 343824 (2013).
Cháfer-Pericás, C. et al. Preliminary case control study to establish the correlation between novel peroxidation biomarkers in cord serum and the severity of hypoxic ischemic encephalopathy. Free Radic. Biol. Med. 97, 244–249 (2016).
Sakamoto, H. et al. Isoprostanes--markers of ischaemia reperfusion injury. Eur. J. Anaesthesiol. 19, 550–559 (2002).
Escobar, J. et al. Development of a reliable method based on ultra-performance liquid chromatography coupled to tandem mass spectrometry to measure thiol-associated oxidative stress in whole blood samples. J. Pharm. Biomed. Anal. 123, 104–112 (2016).
Cháfer-Pericás, C. et al. Ultra high-performance liquid chromatography coupled to tandem mass spectrometry determination of lipid peroxidation biomarkers in newborn serum samples. Anal. Chim. Acta 886, 214–220 (2015).
Cháfer-Pericás, C. et al. Development of a reliable analytical method to determine lipid peroxidation biomarkers in newborn plasma samples. Talanta 153, 152–157 (2016).
García-Flores, L. A. et al. Snapshot situation of oxidative degradation of the nervous system, kidney, and adrenal glands biomarkers-neuroprostane and dihomo-isoprostanes-urinary biomarkers from infancy to elderly adults. Redox Biol. 11, 586–591 (2017).
Cháfer-Pericás, C. et al. Novel biomarkers in amniotic fluid for early assessment of intraamniotic infection. Free Rad. Biol. Med. 89, 734–740 (2015).
García-Blanco, A. et al. References ranges for cortisol and alpha-amylase in mother and newborn saliva samples at different perinatal and postnatal periods. J. Chromatogr. B 1022, 249–255 (2016).
Kuligowski, J. et al. Analysis of lipid peroxidation biomarkers in extremely low gestational age neonates urines by UPLC-MS/MS. Anal. Bioanal. Chem. 406, 4345–4356 (2014).
Kuligowski, J. et al. Urinary lipid peroxidation byproducts: are they relevant for predicting neonatal morbidity in preterm infants. Antioxid. Redox Signal. 23, 178–184 (2015).
van Spronsen, F. J. et al. Key European guidelines for the diagnosis and management of patients with phenylketonuria. Lancet Diabetes Endocrinol. 5, 743–756 (2017).
Bayley, N. Bayley Scales of Infant and Toddler Development – Third Edition (Bayley–III) (Pearson Publishing, San Antonio, 2005).
Wechsler, D. Wechsler Preschool and Primary Scale of Intelligence - Third Edition (WPPSI-III) (Pearson Publishing, San Antonio, 2002).
Wechsler, D. Wechsler Abbreviated Scale ofIntelligence – Second edition (WASI-II) (Pearson Publishing, San Antonio, 2011).
Gioia, G. A., Peter, K., Guy, S. & Kenworthy, L. Behavior Rating Inventory of Executive Functioning (BRIEF) (PAR, Lutz, 2000).
Kamphaus, R. W. The Behavioral Assessment System for Children – Second edition (American Guidance Service, Circle Pines, 2005).
Beck, A. T., Steer, R. A. & Brown, G. Beck Depression Inventory -Second edition (BDI-II) (Pearson Publishing, San Antonio, 1996).
Beck, A. Beck Anxiety Inventory (BAI) 104 (Pearson Publishing, San Antonio, 1993).
Harrison, P. & Oakland, T. Adaptive Behavior Assessment System-Second Edition (ABAS-II) (Pearson Publishing, San Antonio, 2003).
Artuch, R. et al. A longitudinal study of antioxidant status in phenylketonuric patients. Clin. Biochem. 37, 198–203 (2004).
Darling, G. et al. Serum selenium levels in individuals on PKU diets. J. Inher. Metab. Dis. 15, 769–773 (1992).
Gassió, R. et al. Cognitive functions in classic phenylketonuria and mild hyperphenylalaninaemia: experience in a paediatric population. Dev. Med. Child Neurol. 47, 443–448 (2005).
He, Y. Z. et al. The oxidative molecular regulation mechanism of NOX in children with phenylketonuria. Int J. Dev. Neurosci. 38, 178–183 (2014).
Schulpis, K. H., Kariyannis, C. & Papassotiriou, I. Serum levels of neural protein S-100B in phenylketonuria. Clin. Biochem. 37, 76–79 (2004).
Sierra, C. et al. Antioxidant status in hyperphenylalaninemia. Clin. Chim. Acta 276, 1–9 (1998).
Tavana, S., et al. Prooxidant-antioxidant balance in patients with phenylketonuria and its correlation to biochemical and hematological parameters. J. Pediatr. Endocrinol. Metab. 29, 675–680 (2016).
Acknowledgements
M.V. acknowledges a grant from the RETICS funded by the PN 2018-2021 (Spain), ISCIII- Sub-Directorate General for Research Assessment and Promotion and the European Regional Development Fund (FEDER), reference RD16/0022/0001. C.C.-P. acknowledges a postdoctoral “Miguel Servet” grant CP16/00082 from the Health Research Institute Carlos III (Spanish Ministry of Economy, Industry and Innovation). A.G.-B. acknowledges a postdoctoral “Joan Rodés” grant JR17/00003 from the Health Research Institute Carlos III (Spanish Ministry of Economy, Industry and Innovation).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Senior author: Maximo Vento
Rights and permissions
About this article
Cite this article
Rausell, D., García-Blanco, A., Correcher, P. et al. Newly validated biomarkers of brain damage may shed light into the role of oxidative stress in the pathophysiology of neurocognitive impairment in dietary restricted phenylketonuria patients. Pediatr Res 85, 242–250 (2019). https://doi.org/10.1038/s41390-018-0202-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-018-0202-x
This article is cited by
-
Children’s health: insights from space medicine on metabolic health
npj Microgravity (2025)
-
Oxidative stress in phenylketonuria—evidence from human studies and animal models, and possible implications for redox signaling
Metabolic Brain Disease (2021)
-
CRISPR/Cas9 generated knockout mice lacking phenylalanine hydroxylase protein as a novel preclinical model for human phenylketonuria
Scientific Reports (2021)
-
The first study of successful pregnancies in Chinese patients with Phenylketonuria
BMC Pregnancy and Childbirth (2020)
-
Similarities and differences in key diagnosis, treatment, and management approaches for PAH deficiency in the United States and Europe
Orphanet Journal of Rare Diseases (2020)