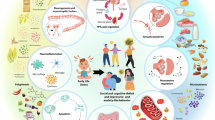
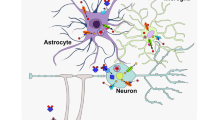
Abstract
Adequate nutrition during the pre- and early-postnatal periods plays a critical role in programming early neurodevelopment. Disruption of neurodevelopment by nutritional deficiencies can result not only in lasting functional deficits, but increased risk of neuropsychiatric disease in adulthood. Historical periods of famine such as the Dutch Hunger Winter and the Chinese Famine have provided foundational evidence for the long-term effects of developmental malnutrition on neuropsychiatric outcomes. Because neurodevelopment is a complex process that consists of many nutrient- and brain-region-specific critical periods, subsequent clinical and pre-clinical studies have aimed to elucidate the specific roles of individual macro- and micronutrient deficiencies in neurodevelopment and neuropsychiatric pathologies. This review will discuss developmental iron deficiency (ID), the most common micronutrient deficiency worldwide, as a paradigm for understanding the role of early-life nutrition in neurodevelopment and risk of neuropsychiatric disease. We will review the epidemiologic data linking ID to neuropsychiatric dysfunction, as well as the underlying structural, cellular, and molecular mechanisms that are thought to underlie these lasting effects. Understanding the mechanisms driving lasting dysfunction and disease risk is critical for development and implementation of nutritional policies aimed at preventing nutritional deficiencies and their long-term sequelae.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Cuthbert, B. N. & Insel, T. R. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 11, 126 (2013).
Georgieff, M. K., Brunette, K. E. & Tran, P. V. Early life nutrition and neural plasticity. Dev. Psychopathol. 27, 411–423 (2015).
González, H. F. & Visentin, S. Micronutrients and neurodevelopment: an update. Arch. Argent. Pediatr. 114, 570–575 (2016).
Cusick, S. E. & Georgieff, M. K. The role of nutrition in brain development: the golden opportunity of the “First 1000 Days”. J. Pediatr. 175, 16–21 (2016).
Krebs, N. F., Lozoff, B. & Georgieff, M. K. Neurodevelopment: the impact of nutrition and inflammation during infancy in low-resource settings. Pediatrics 139, S50–S58 (2017).
Abu-Saad, K. & Fraser, D. Maternal nutrition and birth outcomes. Epidemiol. Rev. 32, 5–25 (2010).
Bale, T. L. et al. Early life programming and neurodevelopmental disorders. Biol. Psychiatry 68, 314–319 (2010).
Davis, J. et al. A review of vulnerability and risks for schizophrenia: beyond the two hit hypothesis. Neurosci. Biobehav. Rev. 65, 185–194 (2016).
Brown, A. S. & Susser, E. S. Prenatal nutritional deficiency and risk of adult schizophrenia. Schizophr. Bull. 34, 1054–1063 (2008).
Susser, E. S. & Lin, S. P. Schizophrenia after prenatal exposure to the Dutch Hunger Winter of 1944-1945. Arch. Gen. Psychiatry 49, 983–988 (1992).
Susser, E. et al. Schizophrenia after prenatal famine. Further evidence. Arch. Gen. Psychiatry 53, 25–31 (1996).
St Clair, D. et al. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959-1961. J. Am. Med. Assoc. 294, 557–562 (2005).
Insel, B. J., Schaefer, C. A., McKeague, I. W., Susser, E. S. & Brown, A. S. Maternal iron deficiency and the risk of schizophrenia in offspring. Arch. Gen. Psychiatry 65, 1136–1144 (2008).
Schmidt, R. J., Tancredi, D. J., Krakowiak, P., Hansen, R. L. & Ozonoff, S. Maternal intake of supplemental iron and risk of autism spectrum disorder. Am. J. Epidemiol. 180, 890–900 (2014).
Sørensen, H. J., Nielsen, P. R., Pedersen, C. B. & Mortensen, P. B. Association between prepartum maternal iron deficiency and offspring risk of schizophrenia: population-based cohort study with linkage of danish national registers. Schizophr. Bull. 37, 982–987 (2011).
Barker, D. J. P. Fetal nutrition and cardiovascular disease in later life. Br. Med. Bull. 53, 96–108 (1997).
Gluckman, P. D. & Hanson, M. A. Living with the past: evolution, development, and patterns of disease. Science 305, 1733–1736 (2004).
Painter, R. C., Roseboom, T. J. & Bleker, O. P. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod. Toxicol. 20, 345–352 (2005).
Lopuhaa, C. E. Atopy, lung function, and obstructive airways disease after prenatal exposure to famine. Thorax 55, 555–561 (2000).
Ravelli, A. et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet 351, 173–177 (1998).
Lumey, L., Stein, A., Kahn, H. & Romijn, J. Lipid profiles in middle-aged men and women after famine exposure during gestation: the Dutch Hunger Winter Families Study. Am. J. Clin. Nutr. 89, 1737–1743 (2009).
Roseboom, T. J. et al. Coronary heart disease after prenatal exposure to the Dutch famine, 1944-45. Heart 84, 595–598 (2000).
Hensch, T. K. Critical period regulation. Annu. Rev. Neurosci. 27, 549–579 (2004).
Clancy, B., Darlington, R. B. & Finlay, B. L. The course of human events: predicting the timing of primate neural development. Dev. Sci. 3, 57–66 (2000).
Holliday, M. in Human Growth: A Comprehensive Treatise (eds Falkner, F. & Tanner, J. M.) 101–117 (Plenium Press, Boston, MA, 1986).
Kretchmer, N., Beard, J. L. & Carlson, S. The role of nutrition in the development of normal cognition. Am. J. Clin. Nutr. 63, 997S–1001S (1996).
McLean, E. et al. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 12, 444 (2009).
Yip, R. Iron deficiency: contemporary scientific issues and international programmatic approaches. J. Nutr. 124, 1479S–1490S (1994).
Mei, Z. et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Am. J. Clin. Nutr. 93, 1312–1320 (2011).
Cogswell, M. E. et al. Assessment of iron deficiency in US preschool children and nonpregnant females of childbearing age: National Health and Nutrition Examination Survey 2003-2006. Am. J. Clin. Nutr. 89, 1334–1342 (2009).
Lozoff, B., Jimenez, E. & Wolf, A. W. Long-term developmental outcome of infants with iron deficiency. N. Engl. J. Med. 325, 687–694 (1991).
Tamura, T. et al. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J. Pediatr. 140, 165–170 (2002).
Lozoff, B., Jimenez, E., Hagen, J., Mollen, E. & Wolf, A. W. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics 105, E51 (2000).
Lukowski, A. F. et al. Iron deficiency in infancy and neurocognitive functioning at 19 years: evidence of long-term deficits in executive function and recognition memory. Nutr. Neurosci. 13, 54–70 (2010).
Lozoff, B. et al. Functional significance of early-life iron deficiency: outcomes at 25 years. J. Pediatr. 163, 1260–1266 (2013).
Corapci, F., Radan, A. E. & Lozoff, B. Iron deficiency in infancy and mother-child interaction at 5 years. J. Dev. Behav. Pediatr. 27, 371–378 (2006).
Chang, S. et al. Iron-deficiency anemia in infancy and social emotional development in preschool-aged Chinese children. Pediatrics 127, e927–e933 (2011).
Lozoff, B. Iron deficiency and child development. Food Nutr. Bull. 28, S560–S571 (2007).
Jorgenson, L. A., Wobken, J. D. & Georgieff, M. K. Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Dev. Neurosci. 25, 412–420 (2003).
Fretham, S. J. B. et al. Temporal manipulation of transferrin-receptor-1-dependent iron uptake identifies a sensitive period in mouse hippocampal neurodevelopment. Hippocampus 22, 1691–1702 (2012).
Tyagi, E., Zhuang, Y., Agrawal, R., Ying, Z. & Gomez-Pinilla, F. Interactive actions of Bdnf methylation and cell metabolism for building neural resilience under the influence of diet. Neurobiol. Dis. 73, 307–318 (2015).
Zeisel, S. Choline, other methyl-donors and epigenetics. Nutrients 9, 445 (2017).
Ly, A. et al. Maternal folic acid supplementation modulates DNA methylation and gene expression in the rat offspring in a gestation period-dependent and organ-specific manner. J. Nutr. Biochem. 33, 103–110 (2016).
Tran, P. V. et al. Prenatal choline supplementation diminishes early-life iron deficiency-induced reprogramming of molecular networks associated with behavioral abnormalities in the adult rat hippocampus. J. Nutr. 146, 484–493 (2016).
Barks, A., Fretham, S. J., Georgieff, M. K. & Tran, P. V. Early-life neuronal-specific iron deficiency alters the adult mouse hippocampal transcriptome. J. Nutr. 148, 1521–1528 (2018).
Mudd, A. T., Fil, J. E., Knight, L. C. & Dilger, R. N. Dietary iron repletion following early-life dietary iron deficiency does not correct regional volumetric or diffusion tensor changes in the developing pig brain. Front. Neurol. 8, 735 (2018).
Leyshon, B. J., Radlowski, E. C., Mudd, A. T., Steelman, A. J. & Johnson, R. W. Postnatal iron deficiency alters brain development in piglets. J. Nutr. 146, 1420–1427 (2016).
Ranade, S. C. et al. Different types of nutritional deficiencies affect different domains of spatial memory function checked in a radial arm maze. Neuroscience 152, 859–866 (2008).
Rao, R., Tkac, I., Schmidt, A. T. & Georgieff, M. K. Fetal and neonatal iron deficiency causes volume loss and alters the neurochemical profile of the adult rat hippocampus. Nutr. Neurosci. 14, 59–65 (2011).
Connor, J. R. & Menzies, S. L. Relationship of iron to oligodendrocytes and myelination. Glia 17, 83–93 (1996).
Brunette, K. E., Tran, P. V., Wobken, J. D., Carlson, E. S. & Georgieff, M. K. Gestational and neonatal iron deficiency alters apical dendrite structure of CA1 pyramidal neurons in adult rat hippocampus. Dev. Neurosci. 32, 238–248 (2010).
Carlson, E. S. et al. Iron is essential for neuron development and memory function in mouse hippocampus. J. Nutr. 139, 672–679 (2009).
Bastian, T. W., von Hohenberg, W. C., Mickelson, D. J., Lanier, L. M. & Georgieff, M. K. Iron deficiency impairs developing hippocampal neuron gene expression, energy metabolism, and dendrite complexity. Dev. Neurosci. 38, 264–276 (2016).
Greminger, A. R., Lee, D. L., Shrager, P. & Mayer-Proschel, M. Gestational iron deficiency differentially alters the structure and function of white and gray matter brain regions of developing rats. J. Nutr. 144, 1058–1066 (2014).
Monk, C. et al. Maternal prenatal iron status and tissue organization in the neonatal brain. Pediatr. Res. 79, 482–488 (2016).
Ball, G. et al. Development of cortical microstructure in the preterm human brain. Proc. Natl Acad. Sci. USA 110, 9541–9546 (2013).
Kroenke, C. D. et al. Microstructural changes of the baboon cerebral cortex during gestational development reflected in magnetic resonance imaging diffusion anisotropy. J. Neurosci. 27, 12506–12515 (2007).
Fukumitsu, K. et al. Synergistic action of dendritic mitochondria and creatine kinase maintains ATP homeostasis and actin dynamics in growing neuronal dendrites. J. Neurosci. 35, 5707–5723 (2015).
Oruganty-Das, A., Ng, T., Udagawa, T., Goh, E. L. K. & Richter, J. D. Translational control of mitochondrial energy production mediates neuron morphogenesis. Cell. Metab. 16, 789–800 (2012).
Dallman, P. R. Biochemical basis for the manifestations of iron deficiency. Annu. Rev. Nutr. 6, 13–40 (1986).
Connor, J. R., Pavlick, G., Karli, D., Menzies, S. L. & Palmer, C. A histochemical study of iron-positive cells in the developing rat brain. J. Comp. Neurol. 355, 111–123 (1995).
Taylor, E. M. & Morgan, E. H. Developmental changes in transferrin and iron uptake by the brain in the rat. Dev. Brain Res. 55, 35–42 (1990).
Morath, D. J. & Mayer-Pröschel, M. Iron modulates the differentiation of a distinct population of glial precursor cells into oligodendrocytes. Dev. Biol. 237, 232–243 (2001).
Morath, D. J. & Mayer-Pröschel, M. Iron deficiency during embryogenesis and consequences for oligodendrocyte generation in vivo. Dev. Neurosci. 24, 197–207 (2002).
Greminger, A. R. & Mayer-Pröschel, M. Identifying the threshold of iron deficiency in the central nervous system of the rat by the auditory brainstem response. ASN Neuro 7, 1–10 (2015).
Lisman, J. E. et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 31, 234–242 (2008).
Beard, J., Erikson, K. M. & Jones, B. C. Neonatal iron deficiency results in irreversible changes in dopamine function in rats. J. Nutr. 133, 1174–1179 (2003).
Pisansky, M. T. et al. Iron deficiency with or without anemia impairs prepulse inhibition of the startle reflex. Hippocampus 23, 952–962 (2013).
Schmidt, A. T., Waldow, K. J., Grove, W. M., Salinas, J. A. & Georgieff, M. K. Dissociating the long-term effects of fetal/neonatal iron deficiency on three types of learning in the rat. Behav. Neurosci. 121, 475–482 (2007).
Algarin, C. et al. Differences on brain connectivity in adulthood are present in subjects with iron deficiency anemia in infancy. Front. Aging Neurosci. 9, 54 (2017).
Buckner, R. L., Andrews-Hanna, J. R. & Schacter, D. L. The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 1124, 1–38 (2008).
Carlson, E. S., Stead, J. D. H., Neal, C. R., Petryk, A. & Georgieff, M. K. Perinatal iron deficiency results in altered developmental expression of genes mediating energy metabolism and neuronal morphogenesis in hippocampus. Hippocampus 17, 679–691 (2007).
Schachtschneider, K. M. et al. Impact of neonatal iron deficiency on hippocampal DNA methylation and gene transcription in a porcine biomedical model of cognitive development. BMC Genom. 17, 856 (2016).
Lubin, F. D., Roth, T. L. & Sweatt, J. D. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J. Neurosci. 28, 10576–10586 (2008).
McGowan, P. O. et al. Broad epigenetic signature of maternal care in the brain of adult rats. PLoS ONE 6, e14739 (2011).
Anderson, O. S. et al. Epigenetic responses following maternal dietary exposure to physiologically relevant levels of bisphenol A. Environ. Mol. Mutagen. 53, 334–342 (2012).
McBirney, M. et al. Atrazine induced epigenetic transgenerational inheritance of disease, lean phenotype and sperm epimutation pathology biomarkers. PLoS ONE 12, e0184306 (2017).
Perkins, A., Lehmann, C., Lawrence, R. C. & Kelly, S. J. Alcohol exposure during development: Impact on the epigenome. Int. J. Dev. Neurosci. 31, 391–397 (2013).
Ke, X. et al. IUGR disrupts the PPARγ-Setd8-H4K20me1and Wnt signaling pathways in the juvenile rat hippocampus. Int. J. Dev. Neurosci. 38, 59–67 (2014).
Tran, P. V., Kennedy, B. C., Lien, Y.-C., Simmons, R. A. & Georgieff, M. K. Fetal iron deficiency induces chromatin remodeling at the Bdnf locus in adult rat hippocampus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 308, R276–R282 (2015).
Tran, P. V., Carlson, E. S., Fretham, S. J. B. & Georgieff, M. K. Early-life iron deficiency anemia alters neurotrophic factor expression and hippocampal neuron differentiation in male rats. J. Nutr. 138, 2495–2501 (2008).
Tran, P. V., Fretham, S. J. B., Carlson, E. S. & Georgieff, M. K. Long-term reduction of hippocampal brain-derived neurotrophic factor activity after fetal-neonatal iron deficiency in adult rats. Pediatr. Res. 65, 493–498 (2009).
Moore, L. D., Le, T. & Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 38, 23–38 (2013).
Tahiliani, M. et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324, 930–935 (2009).
Bachman, M. et al. 5-Hydroxymethylcytosine is a predominantly stable DNA modification. Nat. Chem. 6, 1049–1055 (2014).
Wen, L. & Tang, F. Genomic distribution and possible functions of DNA hydroxymethylation in the brain. Genomics 104, 341–346 (2014).
Chen, Z. et al. Structural insights into histone demethylation by JMJD2 family members. Cell 125, 691–702 (2006).
Tsukada, Y. et al. Histone demethylation by a family of JmjC domain-containing proteins. Nature 439, 811–816 (2006).
Shimamoto, C. et al. Functional characterization of FABP3, 5 and 7 gene variants identified in schizophrenia and autism spectrum disorder and mouse behavioral studies. Hum. Mol. Genet. 23, 6495–6511 (2014).
Picker, J. D. & Coyle, J. T. Do maternal folate and homocysteine levels play a role in neurodevelopmental processes that increase risk for schizophrenia? Harv. Rev. Psychiatry 13, 197–205 (2005).
Schmidt, R. J. et al. Combined prenatal pesticide exposure and folic acid intake in relation to autism spectrum disorder. Environ. Health Perspect. 125, 097007 (2017).
McKee, S. E. & Reyes, T. M. Effect of supplementation with methyl-donor nutrients on neurodevelopment and cognition: considerations for future research. Nutr. Rev. 76, 497–511 (2018).
Wozniak, J. R. et al. Choline supplementation in children with fetal alcohol spectrum disorders: a randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 102, 1113–1125 (2015).
Xiong, X. et al. Impact of pregnancy-induced hypertension on fetal growth. Am. J. Obstet. Gynecol. 180, 207–213 (1999).
Chockalingam, U. M., Murphy, E., Ophoven, J. C., Weisdorf, S. A. & Georgieff, M. K. Cord transferrin and ferritin values in newborn infants at risk for prenatal uteroplacental insufficiency and chronic hypoxia. J. Pediatr. 111, 283–286 (1987).
Bosley, A. R., Sibert, J. R. & Newcombe, R. G. Effects of maternal smoking on fetal growth and nutrition. Arch. Dis. Child. 56, 727–729 (1981).
Sullivan, E. L., Nousen, E. K. & Chamlou, K. A. Maternal high fat diet consumption during the perinatal period programs offspring behavior. Physiol. Behav. 123, 236–242 (2014).
Shen, Y. et al. Associations among maternal pre-pregnancy body mass index, gestational weight gain and risk of autism in the Han Chinese population. BMC Psychiatry 18, 11 (2018).
Monk, C., Georgieff, M. K. & Osterholm, E. A. Maternal prenatal distress and poor nutrition - mutually influencing risk factors affecting infant neurocognitive development. J. Child Psychol. Psychiatry 54, 115–130 (2013).
Shorter, K. R., Felder, M. R. & Vrana, P. B. Consequences of dietary methyl donor supplements: Is more always better? Prog. Biophys. Mol. Biol. 118, 14–20 (2015).
Pylipow, M. et al. Early postnatal weight gain, intellectual performance, and body mass index at 7 years of age in term infants with intrauterine growth restriction. J. Pediatr. 154, 201–206 (2009).
Laus, M. F., Vales, L. D., Costa, T. M. & Almeida, S. S. Early postnatal protein-calorie malnutrition and cognition: a review of human and animal studies. Int. J. Environ. Res. Public. Health 8, 590–612 (2011).
Galler, J. R. et al. Early childhood malnutrition predicts depressive symptoms at ages 11-17. J. Child Psychol. Psychiatry 51, 789–798 (2010).
Morse, N. L. Benefits of docosahexaenoic acid, folic acid, vitamin D and iodine on foetal and infant brain development and function following maternal supplementation during pregnancy and lactation. Nutrients 4, 799–840 (2012).
Brenna, J. T. Long-chain polyunsaturated fatty acids and the preterm infant: a case study in developmentally sensitive nutrient needs in the United States. Am. J. Clin. Nutr. 103, 606s–615s (2016).
Dey, A. C. et al. Maternal and neonatal serum zinc level and its relationship with neural tube defects. J. Health Popul. Nutr. 28, 343–350 (2010).
Fuglestad, A. J., Kroupina, M. G., Johnson, D. E. & Georgieff, M. K. Micronutrient status and neurodevelopment in internationally adopted children. Acta Pediatr. 105, e67–e76 (2016).
Acknowledgements
This work was supported by grants from the National Institutes of Health (R01HD29421 to M.K.G.; R01NS099178 to P.V.T.; F30HD093285 to A.K.B.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barks, A., Hall, A.M., Tran, P.V. et al. Iron as a model nutrient for understanding the nutritional origins of neuropsychiatric disease. Pediatr Res 85, 176–182 (2019). https://doi.org/10.1038/s41390-018-0204-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-018-0204-8
This article is cited by
-
Chromatin accessibility and H3K9me3 landscapes reveal long-term epigenetic effects of fetal-neonatal iron deficiency in rat hippocampus
BMC Genomics (2024)
-
The relationship between childhood hunger experiences and activities of daily living disability: a mediating role of depression
BMC Public Health (2024)
-
Micronutrients and Brain Development
Current Nutrition Reports (2019)