Abstract
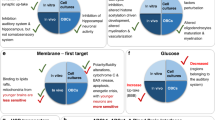
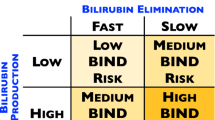
Neonatal hyperbilirubinemia is one of the most frequent diagnoses made in neonates. A high level of unconjugated bilirubin that is unbound to albumin is neurotoxic when the level exceeds age-specific thresholds or at lower levels in neonates with neurotoxic risk factors. Lower range of unbound bilirubin results in apoptosis, while moderate-to-high levels result in neuronal necrosis. Basal ganglia and various brain stem nuclei are more susceptible to bilirubin toxicity. Proposed mechanisms of bilirubin-induced neurotoxicity include excessive release of glutamate, mitochondrial energy failure, release of proinflammatory cytokines, and increased intracellular calcium concentration. These mechanisms are similar to the events that occur following hypoxic–ischemic insult in neonates. Severe hyperbilirubinemia in term neonates has been shown to be associated with increased risk for autism spectrum disorders. The neuropathological finding of bilirubin-induced neurotoxicity also includes cerebellar injury with a decreased number of Purkinje cells, and disruption of multisensory feedback loop between cerebellum and cortical neurons which may explain the clinical characteristics of autism spectrum disorders. Severe hyperbilirubinemia occurs more frequently in infants from low- and middle-income countries (LMIC). Simple devices to measure bilirubin, and timely treatment are essential to reduce neurotoxicity, and improve outcomes for thousands of neonates around the world.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bhutani, V. K., Johnson, L. & Sivieri, E. M. Predictive ability of a pre-discharge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics 103, 6–14 (1999).
Newman, T. B. et al. Jaundice and the infant study team. Outcomes among newborns with total serum bilirubin levels of 25 mg/dL or more. N. Engl. J. Med. 354, 1889–1900 (2006).
Bhutani, V. K. & Johnson, L. H. Urgent clinical need for accurate and precise bilirubin measurements in the United States to prevent kernicterus. Clin. Chem. 50, 477–480 (2004).
Shapiro, S. Bilirubin toxicity in the developing nervous system. Pediatr. Neurol. 29, 410–421 (2003).
Sgro, M., Campbell, D. M., Kandasamy, S. & Shah, V. Incidence of chronic bilirubin encephalopathy in Canada 2007-2008. Pediatrics 130, e886–e890 (2012).
Manning, D., Todd, P., Maxwell, M. & Jane, P. M. Prospective surveillance study of severe hyperbilirubinemia in the newborn in the UK and Ireland. Arch. Dis. Child. Fetal Neonatal Ed. 92, F342–F346 (2007).
Ebbesen, F., Bjerre, J. V. & Vandborg, P. K. Relation between serum bilirubin levels ≥ 450 lmol⁄L and bilirubin encephalopathy; a Danish population-based study. Acta Pædiatr 101, 384–389 (2012).
Greco, C. et al. Neonatal jaundice in low- and middle-income countries: lessons and future directions from the 2015 Don Ostrow Trieste Yellow Retreat. Neonatology 110, 172–180 (2016).
Johnson, L., Bhutani, V. K., Karo, K., Sivieri, E. M. & Shapiro, S. M. Clinical report from the Pilot USA Registry. J. Perinatol. 29, S25–S45 (2009).
Johnson, L. & Bhutani, V. K. The clinical syndrome of bilirubin induced neurologic dysfunction. Semin. Perinatol. 35, 101–113 (2011).
Rose, J. & Vassar, R. Movement disorders due to bilirubin toxicity. Semin. Fetal Neonat. Med. 20, 20–25 (2015).
Wong, R. J. & Stevenson, D. K. Neonatal hemolysis and risk of bilirubin induced neurologic dysfunction. Semin. Fetal Neonat. Med. 20, 26–30 (2015).
Kaplan, M., Bromiker, R. & Hammerman, C. Hyperbilirubinemia, hemolysis and increased bilirubin toxicity. Semin. Perinatol. 38, 429–437 (2014).
American Academy of Pediatrics Subcommittee on Hyperbilirubinemia.. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 114, 297–316 (2004).
Maisels, J. et al. Hyperbilirubinemia in the newborn infant ≥35 weeks gestation: An update with clarifications. Pediatrics 124, 1193–1198 (2009).
Bhutani, V. K., Vilms, R. J. & Hammerman-Johnson, L. Universal bilirubin screening for severe neonatal hyperbilirubinemia. J. Perinatol. 30, S6–S15 (2010).
Kuzniewicz, M. W., Escobar, G. J. & Newman, T. B. Impact of universal bilirubin screening on severe hyperbilirubinemia and phototherapy use. Pediatrics 124, 1031–1039 (2009).
Trikalinos, T. A., Ching, M., Lau, J. & Ip, S. Systematic review of screening for bilirubin encephalopathy in neonates. Pediatrics 124, 1162–1171 (2009).
Kaplan, M., Bromiker, R. & Hammerman, C. Severe neonatal hyperbilirubinemia and kernicterus: are these still problems in the third millennium? Neonatology 100, 354–362 (2011).
Maisels, M. J. Neonatal jaundice. Pediatr. Rev. 27, 443–454 (2006).
Stevenson, D. K., Vreman, H. J. & Wong, R. J. Bilirubin production and the risk of neurotoxicity. Semin. Perinatol. 35, 212–126 (2011).
Slusher, T. M. et al. Burden of severe neonatal jaundice: a systematic review and meta-analysis. BMJ Paediatr. Open 1, e000105 (2017).
De Carvalho, M., Robertson, S. & Klaus, M. Fecal bilirubin excretion and serum bilirubin concentrations in breastfed and bottle-fed infants. J. Pediatr. 107, 786–790 (1985).
Gourley, G. R., Kreamer, B. & Arend, R. The effect of diet on feces and jaundice during the first 3 weeks of life. Gastroenterology 103, 660–667 (1992).
Falcao, A. S. et al. Apoptosis and impairment of neurite network by short exposure of immature rat cortical neurons to unconjugated bilirubin increase with cell differentiation and are additionally enhanced by an inflammatory stimulus. J. Neurosci. Res. 85, 1229–1239 (2007).
Vaz, A. R. et al. Bilirubin selectively inhibits cytochrome c oxidase activity and induces apoptosis in immature cortical neurons: assessment of the protective effects of glycoursodeoxycholic acid.. J. Neurochem. 112, 55–65 (2010).
Bertini, G., Dani, C., Pezzati, M. & Rubaltelli, F. Prevention of bilirubin encephalopathy. Biol. Neonate. 79, 219–223 (2001).
Volpe, J. J. Bilirubin and Brain Injury Neurology of the Newborn. 5th edn, 619–665 (W. B. Saunders, Philadelphia, 2008).
Brites, D. & Fernandes, A. Bilirubin induced neural impairment: a special focus on myelination, age related windows of susceptibility and associated co-morbidities. Semin Fetal Neonat. Medicine 20, 14–19 (2015).
Brodersen, R. & Stern, L. Deposition of bilirubinic acid in the central nervous system--a hypothesis for the development of kernicterus. Acta Paediatr. Scand. 79, 12–19 (1990).
Brites, D. Bilirubin injury to neurons and glial cells: new players, novel targets and newer insights. Semin. Perinatol. 35, 114–120 (2011).
Silva, R. F., Rodrigues, C. M. & Brites, D. Rat cultured neuronal and glial cells respond differently to toxicity of unconjugated bilirubin. Pediatr. Res. 51, 535–541 (2002).
Usman, F., Diala, U. M., Shapiro, S. M., Le Pichon, J. B. & Slusher, T. M. Acute bilirubin encephalopathy and its progression to kernicterus: current perspectives. Res. Rep. Neonatol. 8, 33–44 (2018).
Ostrow, J. D., Pascolo, L. & Tiribelli, C. Reassessment of the unbound concentrations of unconjugated bilirubin in relation to neurotoxicity in vitro. Pediatr. Res. 54, 98–104 (2003).
Falcao, A. S., Fernandes, A., Brito, M. A., Silva, R. F. & Brites, D. Bilirubin induced inflammatory response, glutamate release and cell death in rat cortical astrocytes are enhanced in younger cells. Neurobiol. Dis. 20, 199–206 (2005).
Fernandes, A., Silva, R. F., Falcao, A. S., Brito, M. A. & Brites, D. Cytokine production, glutamate release and cell death in rat cultured astrocytes treated with unconjugated bilirubin and LPS. J. Neuroimmunol. 153, 64–75 (2004).
Ostrow, J. D., Pascolo, L., Brites, D. & Trielli, C. Molecular basis of bilirubin induced neurotoxicity. Trends Mol. Med. 10, 65–70 (2004).
Shapiro, S. Definition of clinical spectrum of kernicterus and bilirubin induced neurologic dysfunction. J. Perinatol. 25, 54–59 (2005).
Watchko, J. F. Kernicterus and the molecular mechanisms of bilirubin induced CNS injury in the newborns. Neuromolecular. Med. 8, 513–529 (2006).
Brito, M. A., Brites, D. & Butterfield, D. A. A link between hyperbilirubinemia, oxidative stress and injury to neocortical synaptosomes. Brain Res. 1026, 33–43 (2004).
Brites, D. The evolving landscape of neurotoxicity by unconjugated bilirubin: role in glial cells and inflammation. Front. Pharmacol. 3, 88 (2012).
Hansen, T. W., Mathiesen, S. B. & Walaas, S. I. Bilirubin has widespread inhibitory effects on protein phosphorylation. Pediatr. Res. 39, 1072–1077 (1996).
Amin, S. B. & Lamola, A. A. Newborn jaundice technologies: unbound bilirubin and bilirubin binding capacity in neonates. Semin. Perinatol. 35, 134–140 (2011).
Ahlfors, C. E., Amin, S. B. & Parker, A. E. Unbound bilirubin predicts abnormal automated auditory brainstem response in a diverse newborn population. J. Perinatol. 29, 305–309 (2009).
Nakamura, H., Yonetani, M., Uetani, Y., Funato, M. & Lee, Y. Determination of serum unbound bilirubin for prediction of kernicterus in low birth weight infants. Acta Paediatr. Jpn. 34, 642–647 (1992).
Cashore, W. J. & Oh, W. Unbound bilirubin and kernicterus in low birth weight infants. Pediatrics 69, 481–485 (1982).
Amin, S. B. et al. Chronic auditory toxicity in late preterm and term infants with significant hyperbilirubinemia. Pediatrics 140, e20164009 (2017).
Amin, S. B., Wang, H., Laroia, N. & Orlando, M. Unbound bilirubin and auditory neuropathy spectrum disorder in late preterm and term infants with severe jaundice. J. Pediatr. 173, 84–89 (2016).
Haymaker, W., Margles, C. & Penstchew, A. in Kernicterus and Importance in Cerebral Palsy (ed. Swinyard, C. A.) pp. 21–229 (Charles C. Thomas, Springfield, 1961).
Ahdab-Barmada, M. & Moosy, J. The neuropathology of kernicterus in the premature neonate. Diagnostic problems. J. Neuropathol. Exp. Neurol. 43, 45–56 (1984).
Claireaux, A. E. in Pathology of Human Kernicterus (University of Toronto Press, Montreal, 1961).
Watchko, J. F. & Tiribelli, C. Bilirubin induced neurologic damage-mechanisms and management approaches. New Eng. J. Med. 369, 2021–2030 (2013).
Shapiro, S. Chronic bilirubin encephalopathy: diagnosis and outcome. Semin Fetal Neonatal Med 15, 157–163 (2010).
Lunsing, R. J. Subtle bilirubin induced neurodevelopmental dysfunction (BIND) in the term and late preterm infant. Does it exist? Semin. Perinatol. 38, 456–471 (2014).
Bortolussi, G. et al. Impairment of enzymatic antioxidant defenses is associated with bilirubin-induced neuronal cell death in the cerebellum of Ugt1 KO mice. Cell Death Dis. 6, e1739 (2015).
Rhine, W. D., Schmitter, S. P., Yu, A. C., Eng, L. F. & Stevenson, D. K. Bilirubin toxicity and differentiation of cultured astrocytes. J. Perinatol. 19, 206–211 (1999).
Fernandes, A. et al. Bilirubin as a determinant for altered neurogenesis, neuritogenesis, and synaptogenesis. Dev. Neurobiol. 69, 568–62 (2009).
Rodrigues, C. M. P., Sola, S., Silva, R. F. M. & Brites, D. Aging confers different sensitivity to the neurotoxic properties of unconjugated bilirubin. Pediatr. Res. 51, 112–118 (2002).
Watchko, J. F. & Maisels, M. J. The enigma of low bilirubin kernicterus in premature infants. Why does it still occur and is it preventable? Semin. Perinatol. 38, 397 (2014).
Morioka, I., Iwatani, S., Koda, T., Iijima, K. & Nakamura, H. Disorders of bilirubin binding to albumin and bilirubin induced neurologic function. Semin. Fetal Neonat. Med. 20, 31–36 (2015).
Amin, S. B. & Wang, H. Bilirubin albumin binding and unbound unconjugated hyperbilirubinemia in premature infants. J. Pediatr. 192, 47–52 (2018).
Hansen, T. W. R., Maynard, E. C., Cashore, W. J. & Oh, W. Endotoxemia and brain bilirubin in the rat. Biol. Neonate. 63, 171–176 (1993).
Petty, M. A. & Lo, E. H. Junctional complexes of the blood brain barrier: permeability change in neuroinflammation. Prog. Neurobiol. 68, 311–323 (2002).
Graziani, L. J. et al. Neurodevelopment of preterm infants: neonatal neurosonographic and serum bilirubin studies. Pediatrics 89, 229–234 (1992).
Johnson, L., Sarmiento, F., Blanc, W. A. & Day, R. Kernicterus in rats with an inherited deficiency of glucuronyl transferase. Am. J. Dis. Child. 97, 591–608 (1959).
Schutta, H. S. & Johnson, L. Bilirubin encephalopathy in the Gunn rat. A fine structure of the cerebellar cortex. J. Neuropathol. Exp. Neurol. 26, 377–396 (1967).
Schutta, H. S. & Johnson, L. Clinical signs and morphologic abnormalities in Gunn rats treated with Sulfadimethoxine. J. Pediatr. 75, 1070–1079 (1969).
Amin, S. B., Smith, T. & Wang, H. Is neonatal jaundice associated with autism spectrum disorders: a systematic review. J. Autism Dev. Diord 41, 1455–1463 (2011).
Bauman, M. L. & Kemper, T. L. Neuroanatomic observations of the brain in autism: a review and future directions. Int. J. Dev. Neurosci. 23, 183–187 (2005).
Hokkanen, L., Launes, J. & Michelsson, K. Adult neurobehavioral outcome of hyperbilirubinemia in full term neonates-a 30 year prospective follow-up study. PeerJ 2, e294 (2014).
Amin, S. B. Clinical assessment of bilirubin induced neurotoxicity in premature infants. Semin. Perinatol. 28, 340–347 (2004).
Amin, S. B., Charafeddine, L. & Guillet, R. Transient bilirubin encephalopathy and apnea of prematurity in 28-32 weeks gestational age infants. J. Perinatol. 25, 386–390 (2005).
Rubin, R. A., Balow, B. & Fisch, R. O. Neonatal serum bilirubin levels related to cognitive development at ages 4 through 7. J. Pediatr. 94, 601–604 (1979).
Seidman, D. S., et al. Neonatal hyperbilirubinemia and physical and cognitive performance at 17 years of age. Pediatrics 88, 828–833 (1991).
Byers, R. K., Paine, R. S. & Crothers, B. Extrapyramidal cerebral palsy with hearing loss following erythroblastosis. Pediatrics 15, 248–254 (1955).
Ip, S. et al. An evidenced based review of important issues concerning hyperbilirubinemia. Pediatrics 114, e130–e153 (2004).
Shapiro, S. M. & Daymont, M. J. Patterns of kernicterus related to neonatal hyperbilirubinemia and gestational age. Pediatr. Res 53(4 Part2), 398A–399A (2003).
Saluja, S., Agarwal, A., Kier, N. & Amin, S. Auditory neuropathy spectrum disorder in late and preterm infants with severe jaundice. Int. J. Pediatr. Otorhinolaryngol. 74, 1292–1297 (2010).
Wisnowski, J. L., Panigrahy, A., Painter, M. J. & Watchko, J. Magnetic resonance imaging of bilirubin encephalopathy: current limitations and promise. Semin. Perinatol. 38, 422–428 (2014).
Yilmaz, Y. et al. Magnetic resonance imaging findings in patients with severe neonatal indirect hyperbilirubinemia. J. Child Neurol. 16, 452–455 (2001).
Christensen, R. D. & Yaish, H. M. Hemolytic disorders causing severe neonatal hyperbilirubinemia. Clin. Perinatol. 42, 515–527 (2015).
Mollison, P. L. & Cutbush, M. A method of measuring the severity of a series of cases of hemolytic disease of the newborn. Blood 6, 777–788 (1951).
Newman, T. B. & Maisels, M. J. Response to commentaries re: evaluation and treatment of jaundice in term newborn: A kinder, gentler approach. Pediatrics 89, 831–833 (1992).
Watchko, J. F. & Oski, F. A. Bilirubin 20 mg/dL=vigintiphobia. Pediatrics 71, 660–663 (1983).
Bhutani, V. K. & Johnson-Hammerman, L. The clinical syndrome of bilirubin induced neurologic dysfunction. Semin Fetal Neonatal Med. 20, 6–13 (2015).
Wennberg, R. P., Ahlfors, C., Bhutani, V. K., Johnson, L. & Shapiro, S. Toward understanding kernicterus: a challenge to improve the management of jaundiced newborns. Pediatrics 117, 474–485 (2006).
Brites, D. et al. Biological risks, for neurological abnormalities associated with hyperbilirubinemia. J. Perinatol. 29(Suppl), S8–S13 (2009).
Wu, Y. W. et al. Risk for cerebral palsy in infants with total serum bilirubin levels at or above the exchange transfusion threshold: a population-based study. JAMA Pediatr. 163, 239–246 (2015).
Moll, M., Goelz, R., Naegele, T., Wilke, M. & Poets, C. F. Are recommended phototherapy thresholds safe enough for extremely low birth weight infants (ELBW)? A report on 2 ELBW infants with kernicterus despite only moderate hyperbilirubinemia. Neonatology 99, 90–94 (2011).
Engle, W. D., Jackson, G. L. & Engle, N. G. Transcutaneous bilirubinometry. Semin. Perinatol. 38, 438–451 (2014).
Bhutani, V. K. et al. Predischarge screening for severe neonatal hyperbilirubinemia identifies infants who need phototherapy. J. Pediatr. 162, 477–482 (2013).
Kuzniewicz, M. W. et al. Risk factors for severe hyperbilirubinemia among infants with borderline bilirubin levels: A nested case-control study. J. Pediatr. 153, 234–240 (2008).
Flaherman, V. J., Kuzniewicz, M. W., Escobar, G. J. & Newman, T. B. Total serum bilirubin exceeding exchange transfusion thresholds in the setting of universal screening. J. Pediatr. 160, 796–800 (2012).
US Preventive Services Task Force. Screening of infants for hyperbilirubinemia to prevent chronic bilirubin encephalopathy: US Preventive Task Force Recommendation. Pediatrics 124, (1172–1177 (2009).
Mah, M. et al. Reduction of severe hyperbilirubinemia after institution of predischarge bilirubin screening. Pediatrics 125, e1143–e1148 (2010).
Wainer, S., Parmar, S. Allegro, D., Rabi, Y. & Lyon, M. E. Impact of a transcutaneous bilirubinometry program on resource utilization and severe hyperbilirubinemia. Pediatrics 129, 77–86 (2012).
Bhutani, V. K. et al. Extreme hyperbilirubinemia and rescue exchange transfusion in California from 2007-2012. J. Perinatol. 36, 853–857 (2016).
Bhutani, V. K. & Johnson, L. H. Jaundice technologies. Prediction of hyperbilirubinemia in term and near term newborns. J. Perinat. 21(Suppl 1), S76–S82 (2001).
Dennery, P. A., Seidman, D. & Stevenson, D. K. Neonatal hyperbilirubinemia. N. Engl. J. Med. 334, 581–590 (2001).
Stevenson, D. K. et al. Prediction of hyperbilirubinemia in near-term and term infants. Pediatrics 108, 31–39 (2001).
Keren, R. et al. A comparison of alternative risk strategies for predicting significant hyperbilirubinemia in term and near -term infants. Pediatrics 121, e170–e179 (2008).
Maisels, M. J. et al. An approach to the management of hyperbilirubinemia in the preterm infant less than 35 weeks of gestation. J. Perinatol. 32, 660–664 (2012).
Stevenson, D. K., Wong, R. J., Arnold, C. C., Pedroza, C. & Tyson, J. E. Phototherapy and the risk of photooxidative injury in extremely low birth weight infants. Clin. Perinatol. 43, 291–295 (2016).
Hansen, T. The roles of phototherapy in the crash cart approach to extreme neonatal jaundice. Semin. Perinatol. 35, 171–174 (2011).
Wickermasinghe, A. C., Kuzniewicz, M. W., McCullough, C. E. & Newman, T. H. Efficacy of subthreshold newborn phototherapy during the birth hospitalization in preventing readmission for phototherapy. JAMA Pediatr. 172, 378–385 (2018).
Bhutani, V. K. Committee of the Fetus and Newborn. Phototherapy to prevent severe neonatal hyperbilirubinemia in the newborn infants 35 weeks or more of gestation. Pediatrics 128, e1046–e1052 (2011).
Wickremasinghe, A. C. et al. Neonatal phototherapy and infantile cancer. Pediatrics 137, e20151353 (2016).
Newman, T. B. et al. Retrospective cohort study and childhood cancer in Northern California. Pediatrics 137, e20151354 (2016).
Morris, B. H. et al. Aggressive vs. conservative phototherapy for infants with extremely low birth weight. N. Engl. Med 359, 1885–1896 (2008). 18.
Olusanya, B. O., Imam, Z. O., Emokpae, A. A. & Iskander, I. F. Revisiting the criteria for exchange transfusion for severe neonatal hyperbilirubinemia in resource-limited settings. Neonatology 109, 97–104 (2016).
Murki, S. & Kumar, P. Blood exchange transfusion for infants with severe neonatal hyperbilirubinemia. Semin. Perinatol. 35, 175–184 (2011).
Maisels, M. J. Neonatal hyperbilirubinemia and Kernicterus-Not gone but sometimes forgotten. Early Hum. Dev. 85, 727–732 (2009).
Nakagawa, M. et al. Correlation between umbilical cord hemoglobin and rate of jaundice requiring phototherapy in healthy newborns. Pediatr. Int. 57, 626–628 (2015).
Bhutani, V. K. et al. Kernicterus: epidemiological strategies for its prevention through systems-based approaches. J. Perinatol. 24, 650–662 (2004).
Bhutani, V. K., Cline, B. K., Donaldson, K. M. & Vreman, H. J. The need to implement effective phototherapy in resource constrained settings. Semin. Perinatol. 35, 192–19 (2011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cayabyab, R., Ramanathan, R. High unbound bilirubin for age: a neurotoxin with major effects on the developing brain. Pediatr Res 85, 183–190 (2019). https://doi.org/10.1038/s41390-018-0224-4
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-018-0224-4
This article is cited by
-
Efficacy of sialic acid supplementation in early life in autism model rats
Scientific Reports (2025)
-
Spectral analysis with highly collimated mini-LEDs as light sources for quantitative detection of direct bilirubin
Discover Nano (2024)
-
Inhibition of Microglial Activation Ameliorates Inflammation, Reduced Neurogenesis in the hippocampus, and Impaired Brain Function in a Rat Model of Bilirubin Encephalopathy
Journal of Neuroimmune Pharmacology (2024)
-
Bilirubin impairs neuritogenesis and synaptogenesis in NSPCs by downregulating NMDAR-CREB-BDNF signaling
In Vitro Cellular & Developmental Biology - Animal (2024)
-
Noninvasive direct bilirubin detection by spectral analysis of color images using a Mini-LED light source
Discover Nano (2023)