Abstract
Background
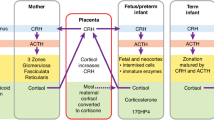
Low birth weight in term-born individuals correlates with adverse cardiometabolic outcomes; excess glucocorticoid exposure has been linked to these relationships. We hypothesized that cortisol and adrenal androgens would correlate inversely with birthweight and directly with markers of cardiometabolic risk in school-aged children born extremely preterm; further, preterm-born would have increased cortisol and adrenal androgens compared to term-born children.
Methods
Saliva samples were obtained at age 6 from 219 preterm-born children followed since birth and 40 term-born children and analyzed for dehydroepiandrosterone (DHEA) and cortisol. Cortisol was also measured at home (awakening, 30′ later, evening).
Results
For preterm-born children, cortisol and DHEA correlated inversely with weight and length Z-scores at 36 weeks PMA and positively with systolic BP. DHEA was higher in preterm-born than term-born children (boys p < 0.01; girls p = 0.04). Cortisol was similar between preterm-born and term-born at study visit; however, preterm-born children showed a blunted morning cortisol. In term-born children, DHEA correlated with BMI (p = 0.04), subscapular, and abdominal skinfold thicknesses (both p < 0.01).
Conclusion
Cortisol and DHEA correlated inversely with early postnatal growth and directly with systolic BP in extremely preterm-born children, suggesting perinatal programming. Blunted morning cortisol may reflect NICU stress, as seen after other adverse childhood experiences (ACEs).
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Barker, D. J. et al. Fetal nutrition and cardiovascular disease in adult life. Lancet 341, 938–941 (1993). Review.
Reynolds, R. M. Glucocorticoid excess and the developmental origins of disease: two decades of testing the hypothesis—–2012 Curt Richter Award Winner. Psychoneuroendocrinology 38, 1–11 (2013). 2013.
Cottrell, E. C., Holmes, M. C., Livingstone, D. E., Kenyon, C. J. & Seckl, J. R. Reconciling the nutritional and glucocorticoid hypotheses of fetal programming. FASEB. J. 26, 1866–1874 (2012).
Economides, D. L., Nicolaides, K. H., Linton, E. A., Perry, L. A. & Chard, T. Plasma cortisol and adrenocorticotropin in appropriate and small for gestational age fetuses. Fetal Ther. 3, 158–164 (1988).
McTernan, C. L. et al. Reduced placental 11beta-hydroxysteroid dehydrogenase type 2 mRNA levels in human pregnancies complicated by intrauterine growth restriction: an analysis of possible mechanisms. J. Clin. Endocrinol. Metab. 86, 4979–4983 (2001).
Ibáñez, L., Potau, N., Marcos, M. V. & de Zegher, F. Exaggerated adrenarche and hyperinsulinism in adolescent girls born small for gestational age. J. Clin. Endocrinol. Metab. 84, 4739–4741 (1999).
Francois, I. & de Zegher, F. Adrenarche and fetal growth. Pediatr. Res. 41, 440–442 (1997).
Ong, K. K. et al. Avon Longitudinal Study of Parents and Children Study Team. Opposing influences of prenatal and postnatal weight gain on adrenarche in normal boys and girls. J. Clin. Endocrinol. Metab. 89, 2647–2651 (2004).
de Jong, F., Monuteaux, M. C., van Elburg, R. M., Gillman, M. W. & Belfort, M. B. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension 59, 226–234 (2012).
Kaseva, N. et al. Blunted hypothalamic-pituitary-adrenal axis and insulin response to psychosocial stress in young adults born preterm at very low birth weight. Clin. Endocrinol. 80, 101–106 (2014).
Meuwese, C. L. et al. Growth-restricted preterm newborns are predisposed to functional adrenal hyperandrogenism in adult life. Eur. J. Endocrinol. 163, 681–689 (2010).
Wells, N. et al. Anthropometric trends from 1997 to 2012 in infants born at ⩽28 weeks’ gestation or less. J. Perinatol. 37, 521–526 (2017).
Donaldson, A., Nicolini, U., Symes, E. K., Rodeck, C. H. & Tannirandorn, Y. Changes in concentrations of cortisol, dehydroepiandrosterone sulphate and progesterone in fetal and maternal serum during pregnancy. Clin. Endocrinol. 35, 447–451 (1991).
Watterberg, K. L. et al. Prophylaxis of early adrenal insufficiency to prevent bronchopulmonary dysplasia: a multicenter trial. Pediatrics 114, 1649–1657 (2004).
Watterberg, K. L. et al. Effect of dose on response to ACTH in extremely low birth weight infants. J. Clin. Endocrinol. Metab. 90, 6380–6385 (2005).
Finer, N. N. et al. Early CPAP versus surfactant in extremely preterm infants. New Engl. J. Med. 362, 1970–1979 (2010).
Hintz, S. R. et al. Preterm neuroimaging and school-age cognitive outcomes. Pediatrics 42, e20174058 (2018).
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation and treatment of high blood pressure in children and adolescents. Pediatrics 114, 555–576 (2004).
Granger, D. A., Schwartz, E. B., Booth, A., Curran, M. & Zakaria, D. Assessing dehydroepiandrosterone in saliva: a simple radioimmunoassay for use in studies of children, adolescents and adults. Psychoneuroendocrinology 24, 567–579 (1999).
Ahn, R. S., Lee, Y. J., Choi, J. Y., Kwon, H. B. & Chun, S. I. Salivary cortisol and DHEA levels in the Korean population: age-related differences, diurnal rhythm, and correlations with serum levels. Yonsei. Med. J. 48, 379–388 (2007).
Granger, D. A. et al. Focus on methodology: salivary bioscience and research on adolescence: an integrated perspective. J. Adolesc. 35, 1081–1095 (2012).
Fenton, T. R. & Kim, J. H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 13:59 (2013).
Moulton, L. H. & Halsey, N. A. A mixture model with detection limits for regression analyses of antibody response to vaccine. Biometrics 51, 1570–1578 (1995).
Voutilainen, R. & Jääskeläinen, J. Premature adrenarche: etiology, clinical findings, and consequences. J. Steroid Biochem. Mol. Biol. 145, 226–236 (2015).
Girgis, R. et al. Ethnic differences in androgens, IGF-I and body fat in healthy prepubertal girls. Pediatr. Endocrinol. Metab. 13, 497–503 (2000).
Finken, M. J. et al. Glucocorticoid programming in very preterm birth. Horm. Res. Paediatr. 85, 221–231 (2016).
Tenhola, S. et al. Increased adrenocortical and adrenomedullary hormonal activity in 12-year-old children born small for gestational age. J. Pediatr. 141, 477–482 (2002).
Victoria, N. C. & Murphy, A. Z. The long-term impact of early life pain on adult responses to anxiety and stress: Historical perspectives and empirical evidence. Exp. Neurol. 275(Pt 2), 261–273 (2016).
Maurer, N. et al. Salivary and hair glucocorticoids and sleep in very preterm children during school age. Psychoneuroendocrinology 72, 166–174 (2016).
Brummelte, S. et al. Cortisol levels in former preterm children at school age are predicted by neonatal procedural pain-related stress. Psychoneuroendocrinology 51, 151–163 (2015).
Grunau, R. E. Neonatal pain in very preterm infants: long-term effects on brain, neurodevelopment and pain reactivity. Rambam Maimonides Med. J. 4, e0025 (2013).
Kalmakis, K. A., Meyer, J. S., Chiodo, L. & Leung, K. Adverse childhood experiences and chronic hypothalamic-pituitary-adrenal activity. Stress 18, 446–450 (2015).
Leneman, K. B., Donzella, B., Desjardins, C. D., Miller, B. S. & Gunnar, M. R. The slope of cortisol from awakening to 30 min post-wake in post-institutionalized children and early adolescents. Psychoneuroendocrinology 96, 93–99 (2018).
Steudte-Schmiedgen, S., Kirschbaum, C., Alexander, N. & Stalder, T. An integrative model linking traumatization, cortisol dysregulation and posttraumatic stress disorder: Insight from recent hair cortisol findings. Neurosci. Biobehav. Rev. 69, 124–135 (2016).
Kerkhof, G. F., Willemsen, R. H., Leunissen, R. W., Breukhoven, P. E. & Hokken-Koelega, A. C. Health profile of young adults born preterm: negative effects of rapid weight gain in early life. J. Clin. Endocrinol. Metab. 97, 4498–4506 (2012).
Huysman, M. W., Hokken-Koelega, A. C., De Ridder, M. A. & Sauer, P. J. Adrenal function in sick very preterm infants. Pediatr. Res. 48, 629–633 (2000).
Ng, P. C. et al. Reference ranges and factors affecting the human corticotropin-releasing hormone test in preterm, very low birth weight infants. J. Clin. Endocrinol. Metab. 87, 4621–4628 (2002).
Xi, B. et al. Can pediatric hypertension criteria be simplified? A prediction analysis of subclinical cardiovascular outcomes from the Bogalusa Heart Study. Hypertension 69, 6sz91–696sz91 (2017).
Corvalán, C., Uauy, R. & Mericq, V. Obesity is positively associated with dehydroepiandrosterone sulfate concentrations at 7 y in Chilean children of normal birth weight. Am. J. Clin. Nutr. 97, 318–325 (2013).
Stoltz Sjöström, E. et al. Nutrient intakes independently affect growth in extremely preterm infants: results from a population-based study. Acta Paediatr. 102, 1067–1074 (2013).
Sanders, M. R. & Hall, S. L. Trauma-informed care in the newborn intensive care unit: promoting safety, security and connectedness. J. Perinatol. 38, 3–10 (2018).
Acknowledgments
The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Heart, Lung, and Blood Institute (NHLBI) provided grant support for the Neonatal Research Network’s Extended Follow-up at School Age for the SUPPORT Neuroimaging and Neurodevelopmental Outcomes (NEURO) Cohort through cooperative agreements. NHLBI provided support for this study (R01HL117764). While NICHD staff had input into the study design, conduct, analysis, and manuscript drafting, the comments and views of the authors do not necessarily represent the views of the NICHD. Data collected at participating sites of the NICHD Neonatal Research Network (NRN) were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed and analyzed the data for this study. On behalf of the NRN, Drs. Abhik Das (DCC Principal Investigator), Marie Gantz, Lisa Wrage, and Helen Cheng (DCC Statisticians) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
Author information
Authors and Affiliations
Consortia
Contributions
K.W. made substantial contributions to conception and design of the study, acquisition, analysis, and interpretation of data. She drafted the initial manuscript, revised it, and has given final approval of the version to be published. S.H. provided resources for the study, made substantial contributions to the investigation, reviewed and edited the manuscript, and has given final approval of the version to be published. B.D. made substantial contributions to the formal analysis and interpretation of the data, writing and review of the manuscript, and has given final approval of the version to be published. B.V. made substantial contribution to the investigation, review and editing of the manuscript, and has given final approval of the version to be published. J.L. made substantial contribution to the methodology and investigation, reviewed and edited the manuscript, and has given final approval of the version to be published. J.N. made substantial contribution to the study methodology, provided supervision/oversight, reviewed and edited the manuscript, and has given final approval of the version to be published. D.W. made substantial contribution to conception and design of the study and its methodology, data curation and analysis, review and editing of the manuscript, and has given final approval of the version to be published. C.L. made substantial contribution to the conception, design and methodology of the study, provided supervision and oversight, reviewed and edited the manuscript, and has given final approval of the version to be published. E.D. made substantial contribution to the methodology of the study, reviewed and edited the manuscript, and has given final approval of the version to be published. D.G. made substantial contribution to the study methodology and data curation, provided supervision/oversight, participated in writing and editing of the manuscript, and has given final approval of the version to be published. S.S. provided substantial contribution to the study methodology, provided supervision/oversight, reviewed and edited the manuscript, and has given final approval of the version to be published. A.P. provided substantial contribution to the study investigation, participated in writing and editing of the manuscript, and has given final approval of the version to be published. R.H. provided substantial contribution to the conception and design of the study, supervision/oversight for the investigation, review and editing of the manuscript, and has given final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
D.A.G. is founder and chief scientific and strategy advisor at Salimetrics LLC and Salivabio LLC. The nature of those relationships is managed by the policies of the committees on conflict of interest at the Johns Hopkins University School of Medicine and the University of California at Irvine. All the remaining authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A complete list of non-author contributors appears in Appendix.
Appendix
Appendix
NRN Steering Committee Chairs
Alan H. Jobe, MD PhD, University of Cincinnati (2003-2006).
Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine (2006–2011).
Richard A. Polin, MD, Division of Neonatology, College of Physicians and Surgeons, Columbia University, (2011-present).
Abbot R. Laptook, MD; Angelita M. Hensman, MS RNC-NIC; Elisa Vieira, RN BSN; Emilee Little, RN BSN; Katharine Johnson, MD; Barbara Alksninis, PNP; Mary Lenore Keszler, MD; Andrea M. Knoll; Theresa M. Leach, MEd CAES; Elisabeth C. McGowan, MD; Victoria E. Watson, MS CAS—Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904).
Michele C. Walsh, MD MS; Avroy A. Fanaroff, MD; Deanne E. Wilson-Costello, MD; Nancy S. Newman, RN; Bonnie S. Siner, RN; Arlene Zadell, RN; Julie DiFiore, BS; Monika Bhola, MD; Harriet G. Friedman, MA; Gulgun Yalcinkaya, MD—Case Western Reserve University, Rainbow Babies & Children’s Hospital (U10 HD21364, M01 RR80).
Ronald N. Goldberg, MD; C. Michael Cotten, MD MHS; Kathryn E. Gustafson, PhD; Ricki F. Goldstein, MD; Patricia Ashley, MD; Kathy J. Auten, MSHS; Kimberley A. Fisher, PhD FNP-BC IBCLC; Katherine A. Foy, RN; Sharon F. Freedman, MD; Melody B. Lohmeyer, RN MSN; William F. Malcolm, MD; David K. Wallace, MD MPH—Duke University School of Medicine, University Hospital, and Duke Regional Hospital (U10 HD40492, M01 RR30).
David P. Carlton, MD; Barbara J. Stoll, MD; Ira Adams-Chapman, MD; Susie Buchter, MD; Anthony J. Piazza, MD; Sheena Carter, PhD; Sobha Fritz, PhD; Ellen C. Hale, RN BS CCRC; Amy K. Hutchinson, MD; Maureen Mulligan LaRossa, RN; Yvonne Loggins, RN, Diane Bottcher, RN—Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory Crawford Long Hospital (U10 HD27851, RR25008, M01 RR39).
Stephanie Wilson Archer, MA—Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Brenda B. Poindexter, MD MS; Gregory M. Sokol, MD; Heidi M. Harmon, MD MS; Lu-Ann Papile, MD; Abbey C. Hines, PsyD; Leslie D. Wilson, BSN CCRC; Dianne E. Herron, RN; Lucy Smiley, CCRC—Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (U10 HD27856, M01 RR750).
Kathleen A. Kennedy, MD MPH; Jon E. Tyson, MD MPH; Andrea F. Duncan, MD MSClinRes; Allison G. Dempsey, PhD; Janice John, CPNP; Patrick M. Jones, MD MA; M. Layne Lillie, RN BSN; Saba Siddiki, MD; Daniel K. Sperry, RN—McGovern Medical School at The University of Texas Health Science Center at Houston and Children’s Memorial Hermann Hospital (U10 HD21373).
Carol J. Blaisdell, MD, Victoria Pemberton RNC, MS, CCRC—National Heart, Lung, and Blood Institute.
Abhik Das, PhD; Dennis Wallace, PhD; Marie G. Gantz, PhD; Jeanette O’Donnell Auman, BS; Jane A. Hammond, PhD; W. Kenneth Poole, PhD (deceased)—RTI International (U10 HD36790).
Krisa P. Van Meurs, MD; David K. Stevenson, MD; M. Bethany Ball, BS CCRC; Maria Elena DeAnda, PhD; Gabrielle T. Goodlin, BAS—Stanford University and Lucile Packard Children’s Hospital (U10 HD27880, UL1 RR25744, M01 RR70).
Ivan D. Frantz III, MD; John M. Fiascone, MD; Elisabeth C. McGowan, MD; Anne Kurfiss, MPH; Brenda L. MacKinnon, RNC; Ellen Nylen, RN BSN; Ana Brussa, MS OTR/L; Cecelia Sibley, PT MHA—Tufts Medical Center, Floating Hospital for Children (U10 HD53119, M01 RR54).
Waldemar A. Carlo, MD; Namasivayam Ambalavanan, MD; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN; Vivien A. Phillips, RN BSN; Kristy Domanovich, PhD; Sally Whitley, MA OTR-L FAOTA; Leigh Ann Smith, CRNP; Carin R. Kiser, MD—University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216, M01 RR32).
Neil N. Finer, MD; Donna Garey, MD; Maynard R. Rasmussen; MD; Paul R. Wozniak, MD; Yvonne E. Vaucher, MD MPH; Martha G. Fuller, PhD RN; Natacha Akshoomoff, PhD; Wade Rich, BSHS RRT; Kathy Arnell, RNC; Renee Bridge, RN—University of California – San Diego Medical Center and Sharp Mary Birch Hospital for Women (U10 HD40461).
Edward F. Bell, MD; Tarah T. Colaizy, MD MPH; John A. Widness, MD; Jonathan M. Klein, MD; Karen J. Johnson, RN BSN; Michael J. Acarregui, MD; Diane L. Eastman, RN CPNP MA; Tammy L. V. Wilgenbusch, PhD—University of Iowa (U10 HD53109, UL1 TR442, M01 RR59).
Robin K. Ohls, MD; Janell Fuller, MD; Conra Backstrom Lacy, RN; Rebecca A. Thomson, RN BSN; Sandra Brown, RN BSN—University of New Mexico Health Sciences Center (R01 HL117764, U10 HD53089, M01 RR997).
Pablo J. Sánchez, MD; Roy J. Heyne, MD; Charles R. Rosenfeld, MD; Walid A. Salhab, MD; Luc Brion, MD; Sally S. Adams, MS RN CPNP; James Allen, RRT; Laura Grau, RN; Alicia Guzman; Gaynelle Hensley, RN; Elizabeth T. Heyne, PsyD PA-C; Jackie F. Hickman, RN; Lizette E. Lee, RN; Melissa H. Leps, RN; Linda A. Madden, RN CPNP; Melissa Swensen Martin, RN; Nancy A. Miller, RN; Janet S. Morgan, RN; Araceli Solis, RRT; Catherine Twell Boatman, MS CIMI; Diana M Vasil, MSN BSN RNC-NIC—University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children’s Medical Center Dallas (U10 HD40689, M01 RR633).
Bradley A. Yoder, MD; Roger G. Faix, MD; Shawna Baker, RN; Karen A. Osborne, RN BSN CCRC; Carrie A. Rau, RN BSN CCRC; Sarah Winter, MD; Sean D. Cunningham, PhD; Ariel C. Ford, PsyD—University of Utah Medical Center, Intermountain Medical Center, LDS Hospital, and Primary Children’s Medical Center (U10 HD53124, M01 RR64).
Athina Pappas, MD; Beena G. Sood, MD MS; Rebecca Bara, RN BSN; Thomas L. Slovis, MD (deceased); Laura A. Goldston, MA; Mary Johnson, RN BSN—Wayne State University, Hutzel Women’s Hospital, and Children’s Hospital of Michigan (U10 HD21385).
Rights and permissions
About this article
Cite this article
Watterberg, K.L., Hintz, S.R., Do, B. et al. Adrenal function links to early postnatal growth and blood pressure at age 6 in children born extremely preterm. Pediatr Res 86, 339–347 (2019). https://doi.org/10.1038/s41390-018-0243-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-018-0243-1
This article is cited by
-
Kidney health outcomes in children born very prematurely compared to full-term counterparts: a systematic review and meta-analysis
Pediatric Nephrology (2025)
-
Early detection of kidney impairment in school-aged children born very preterm: a parallel use of traditional and modern biomarkers
Pediatric Nephrology (2025)
-
Impact of postnatal steroids on peripheral avascular retina and severity of retinopathy of prematurity
Pediatric Research (2023)
-
Cortisol awakening response and developmental outcomes at 6–7 years in children born extremely preterm
Pediatric Research (2023)
-
DNA methylation in former extremely low birth weight newborns: association with cardiovascular and endocrine function
Pediatric Research (2022)