Abstract
Background
Newborn pigs offer theoretical advantages for studying newborn hypoxic–ischemic (HI) brain damage because of a development and structure similar to the human brain. However, the correlation between functional features and actual HI brain damage has not been reported.
Methods
Newborn pigs were examined daily for 3 days after a HI insult using amplitude-integrated EEG (aEEG), and a neurobehavioral score enriched with stress and social and object interaction-driven activity evaluation. Brain damage was then assessed using histologic, immunohistochemical, and proton magnetic resonance spectroscopy studies. Brain concentration of several neurotransmitters was determined by HPLC.
Results
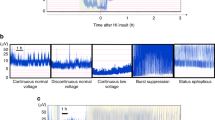
HI insult led to aEEG amplitude decrease, muscle tone and activity impairment, eating disorders, poor environmental interaction, and increased motionless periods. Basal aEEG amplitude, muscle tone, and general behavior were the best predictive items for histological and biochemical (lactate/N-acetylaspartate ratio) brain damage. Hyperexcitable response to stress correlated inversely with brain damage. Motionless time, which correlated with brain damage severity, was inversely related to brain concentration of dopamine and norepinephrine.
Conclusion
Standard neurologic examination of brain activity and motor and behavioral performance of newborn pigs is a valuable tool to assess HI brain damage, thus offering a powerful translational model for HI brain damage pathophysiology and management studies.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Vexler, Z. S. et al. Translational stroke research in the developing brain. Pediatr. Neurol. 34, 459–463 (2006).
Gieling, E. T., Nordquist, R. E. & van der Staay, F. J. Assessing learning and memory in pigs. Anim. Cogn. 14, 151–173 (2011).
Mudd, A. T. & Dilger, R. N. Early-life nutrition and neurodevelopment : use of the piglet as a translational model. Adv. Nutr. 8, 92–104 (2017).
Naim, M. Y. et al. Folic acid enhances early functional recovery in a piglet model of pediatric head injury. Dev. Neurosci. 32, 466–479 (2011).
Conrad, M. S. & Johnson, R. W. The domestic piglet: an important model for investigating the neurodevelopmental consequences of early life insults. Annu Rev. Anim. Biosci. 3, 245–264 (2015).
Alvarez, F. J. et al. Neuroprotective effects of the nonpsychoactive cannabinoid cannabidiol in hypoxic-ischemic newborn piglets. Pediatr. Res. 64, 653–658 (2008).
Lafuente, H. et al. Cannabidiol reduces brain damage and improves functional recovery after acute hypoxia-ischemia in newborn pigs. Pediatr. Res. 70, 272–277 (2011).
Pazos, M. R. et al. Mechanisms of cannabidiol neuroprotection in hypoxic-ischemic newborn pigs: role of 5HT(1A) and CB2 receptors. Neuropharmacology 71, 282–291 (2013).
Arruza, L. et al. Hypoxic-ischemic brain damage induces distant inflammatory lung injury in newborn piglets. Pediatr. Res. 79, 401–408 (2015).
LeBlanc, M. H. et al. MK-801 does not protect against hypoxic-ischemic brain injury in piglets. Stroke 22, 1270–1275 (1991).
Temesvári, P. et al. Impaired early neurologic outcome in newborn piglets reoxygenated with 100% oxygen compared with room air after pneumothorax-induced asphyxia. Pediatr. Res. 49, 812–819 (2001).
Schubert, S. et al. Neuroprotective effects of topiramate after hypoxia-ischemia in newborn piglets. Brain Res. 1058, 129–136 (2005).
Provencher, S. W. Automatic quantitation of localized in vivo1H spectra with LC Model. NMR Biomed. 14, 260–264 (2001).
Adell, a & Artigas, F. A microdialysis study of the in vivo release of 5-HT in the median raphe nucleus of the rat. Br. J. Pharmacol. 125, 1361–1367 (1998).
Thayyil, S. et al. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis. Pediatrics 125, e382–e395 (2010). A.
Harris, J. L., Choi, I.-Y. & Brooks, W. M. Probing astrocyte metabolism in vivo: proton magnetic resonance spectroscopy in the injured and aging brain. Front. Aging Neurosci. 7, 202 (2015).
Dilger, R. N. & Johnson, R. W. Behavioral assessment of cognitive function using a translational neonatal piglet model. Brain Behav. Immun. 24, 1156–1165 (2010).
Björkman, S. T., Miller, S. M., Rose, S. E., Burke, C. & Colditz, P. B. Seizures are associated with brain injury severity in a neonatal model of hypoxia-ischemia. Neuroscience 166, 157–167 (2010).
Jacobs, S. E., Hunt, R., Tarnow-Mordi, W. O., Inder, T. E. & Davis, P. G. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 4, CD003311 (2010).
Sullivan, S. M., Björkman, S. T., Miller, S. M., Colditz, P. B. & Pow, D. V. Structural remodeling of gray matter astrocytes in the neonatal pig brain after hypoxia/ischemia. Glia 58, 181–194 (2010).
Ferrazzano, P. et al. Age-dependent microglial activation in immature brains after hypoxia- ischemia. CNS Neurol. Disord. Drug Targets 12, 338–349 (2013).
Juul, S. E. & Ferriero, D. M. Pharmacologic neuroprotective strategies in neonatal brain injury. Clin. Perinatol. 41, 219–231 (2014).
Weeke, L. C. et al. A comparison of the thompson encephalopathy score and amplitude-integrated electroencephalography in infants with perinatal asphyxia and therapeutic hypothermia. Neonatology 112, 24–29 (2017).
Merchant, N. & Azzopardi, D. Early predictors of outcome in infants treated with hypothermia for hypoxic-ischaemic encephalopathy. Dev. Med. Child Neurol. 57, 8–16 (2015).
Korotchikova, I., Stevenson, N. J., Walsh, B. H., Murray, D. M. & Boylan, G. B. Quantitative EEG analysis in neonatal hypoxic ischaemic encephalopathy. Clin. Neurophysiol. 122, 1671–1678 (2011).
Dunne, J. M. et al. Automated electroencephalographic discontinuity in cooled newborns predicts cerebral MRI and neurodevelopmental outcome. Arch. Dis. Child. Fetal Neonatal Ed. 102, F58–F64 (2017).
Loepke, A. W. et al. Desflurane improves neurologic outcome after low-flow cardiopulmonary bypass in newborn pigs. Anesthesiology 97, 1521–1527 (2002).
Sarnat, H. B. & Sarnat, M. S. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch. Neurol. 33, 696–705 (1976).
Thompson, C. M. et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. Int J. Paediatr. 86, 757–761 (1997).
Coleman, M. B. et al. Neonatal neurobehavioral abnormalities and MRI brain injury in encephalopathic newborns treated with hypothermia. Early Hum. Dev. 89, 733–737 (2013).
Martinez-Biarge, M., Diez-Sebastian, J., Rutherford, M. A. & Cowan, F. M. Outcomes after central grey matter injury in term perinatal hypoxic-ischaemic encephalopathy. Early Hum. Dev. 86, 675–682 (2010).
Kanitz, E., Tuchscherer, M., Puppe, B., Tuchscherer, A. & Stabenow, B. Consequences of repeated early isolation in domestic piglets (Sus scrofa) on their behavioural, neuroendocrine, and immunological responses. Brain Behav. Immun. 18, 35–45 (2004).
Hama, S. et al. Neuroanatomic pathways associated with monoaminergic dysregulation after stroke. Int. J. Geriatr. Psychiatry 32, 633–642 (2017).
Meng, G. et al. Predictors of early-onset post-ischemic stroke depression: a cross-sectional study. BMC Neurol. 17, 1–8 (2017).
Acknowledgements
We are indebted to Martin Santos, PhD, and María Dolores Molina Corzo for their help performing this experiment. We also thank Jason Willis-Lee MITI for medical writing assistance during preparation of the final manuscript. This work was supported by grants from the Carlos III Research Institute (ISCiii) according to the Spanish Plan for R + D + I 2008–2011 and the State Plan for Scientific and Technical Research and Innovation 2016–2019, with co-funding from the European Regional Development Funds (FEDER) (FIS- PI16/00689) and from the Biomedicine Program, Community of Madrid (S2010/BMD-2308).
Author information
Authors and Affiliations
Contributions
Substantial contributions to: Conception and design: J.M.-O., F.-J.A. Acquisition of data: L.B., A.C., H.L., J.M.-O. Analysis and interpretation of data: C.V., M.C., L.C., L.J.-S., M.R.P., J.M.-O. Drafting the article or revising it critically for important intellectual content: J.M.-O., F.-J.A. Final approval of the version to be published: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Barata, L., Cabañas, A., Lafuente, H. et al. aEEG and neurologic exam findings correlate with hypoxic–ischemic brain damage severity in a piglet survival model. Pediatr Res 85, 539–545 (2019). https://doi.org/10.1038/s41390-019-0282-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-019-0282-2