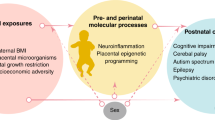
Abstract
The placenta is vital for fetal growth, and compromised function is associated with abnormal development, especially of the brain. Linking placental function to brain development is a new field we have dubbed neuroplacentology. Approximately 380,000 infants in the United States each year abruptly lose placental support upon premature birth, and more than 10% of pregnancies are affected by more insidious placental dysfunction such as preeclampsia or infection. Abnormal fetal brain development or injury can lead to life-long neurological impairments, including psychiatric disorders. The majority of research connecting placental compromise to fetal brain injury has focused on gas exchange or nutritional programming, neglecting the placenta’s essential neuroendocrine role. We will review the current evidence that placental dysfunction, particularly endocrine dysfunction, secretion of pro-inflammatory cytokines, or barrier breakdown may place many thousands of fetuses at risk for life-long neurodevelopmental impairments each year. Understanding how specific placental factors shape brain development and increase the risk for later psychiatric disorders, including autism, attention deficit disorder, and schizophrenia, paves the way for novel treatment strategies to maintain the normal developmental milieu and protect from further injury.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Nosarti, C. et al. Preterm birth and psychiatric disorders in young adult life. Arch. Gen. Psychiatry 69, E1–E8 (2012).
Marin, O. Developmental timing and critical windows for the treatment of psychiatric disorders. Nat. Med. 22, 1229–1238 (2016).
Redline, R. W. Severe fetal placental vascular lesions in term infants with neurologic impairment. Am. J. Obstet. Gynecol. 192, 452–457 (2005).
Barker, D. J. & Osmond, C. Childhood respiratory infection and adult chronic bronchitis in England and Wales. BMJ (Clin. Res. Ed.) 293, 1271–1275 (1986).
Barker, D. J. et al. Weight in infancy and death from ischaemic heart disease. Lancet 2, 577–580 (1989).
Burton, G. J., Fowden, A. L. & Thornburg, K. L. Placental origins of chronic disease. Physiol. Rev. 96, 1509–1565 (2016).
Sallout, B. & Walker, M. The fetal origin of adult diseases. J. Obstet. Gynaecol. 23, 555–560 (2003).
Nair, S. & Salomon, C. Extracellular vesicles and their immunomodulatory functions in pregnancy. Semin. Immunopathol. 40, 425–437 (2018).
Fallen, S. et al. Extracellular vesicle RNAs reflect placenta dysfunction and are a biomarker source for preterm labour. J. Cell Mol. Med. 22, 2760–2773 (2018).
Liu, H. et al. Estimation of the burden of human placental micro- and nano-vesicles extruded into the maternal blood from 8 to 12 weeks of gestation. Placenta 72–73, 41–47 (2018).
Nelson, K. B. & Blair, E. The placenta and neurologic and psychiatric outcomes in the child: study design matters. Placenta 32, 623–625 (2011).
VanWijk, M. J. et al. Vascular function in preeclampsia. Cardiovasc. Res. 47, 38–48 (2000).
Wintermark, P. et al. Placental pathology in asphyxiated newborns meeting the criteria for therapeutic hypothermia. Am. J. Obstet. Gynecol. 203, 579 e571–e579 (2010).
Lachapelle J. et al. Placental pathology in asphyxiated newborns treated with therapeutic hypothermia. J. Neonatal. Perinatal. Med. 8, 33–40 (2015).
Bonifacio, S. L. et al. Extreme premature birth is not associated with impaired development of brain microstructure. J. Pediatr. 157, 726–732 e721 (2010).
Taylor, H. G. & Clark, C. A. Executive function in children born preterm: risk factors and implications for outcome. Semin. Perinatol. 40, 520–529 (2016).
Joseph, R. M. et al. Investigators ES. Neurocognitive and academic outcomes at agosartie 10 years of extremely preterm newborns. Pediatrics 137 (2016). https://doi.org/10.1542/peds.2015-4343e20154343.
Kim, C. J. et al. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am. J. Obstet. Gynecol. 213, S29–S52 (2015).
Romero, R., Dey, S. K. & Fisher, S. J. Preterm labor: one syndrome, many causes. Science 345, 760–765 (2014).
Apel-Sarid, L. et al. Term and preterm (<34 and <37 weeks gestation) placental pathologies associated with fetal growth restriction. Arch. Gynecol. Obstet. 282, 487–492 (2010).
Redline, R. W. et al. Placental lesions as predictors of cerebral palsy and abnormal neurocognitive function at school age in extremely low birth weight infants (<1 kg). Pediatr. Dev. Pathol. 10, 282–292 (2007).
Leviton, A. et al. Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Developmental Epidemiology Network Investigators. Pediatr. Res 46, 566–575 (1999).
Kim, C. J. et al. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am. J. Obstet. Gynecol. 213, S53–S69 (2015).
Faa, G. et al. Fetal programming of neuropsychiatric disorders. Birth Defects Res. C 108, 207–223 (2016).
Elovitz, M. A. et al. Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int. J. Dev. Neurosci. 29, 663–671 (2011).
Ellman, L. M. et al. Structural brain alterations in schizophrenia following fetal exposure to the inflammatory cytokine interleukin-8. Schizophr. Res. 121, 46–54 (2010).
Curtis, D. J. et al. Secretions from placenta, after hypoxia/reoxygenation, can damage developing neurones of brain under experimental conditions. Exp. Neurol. 261, 386–395 (2014).
Patterson, P. H. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav. Brain Res. 204, 313–321 (2009).
Wolff, A. R. & Bilkey, D. K. The maternal immune activation (MIA) model of schizophrenia produces pre-pulse inhibition (PPI) deficits in both juvenile and adult rats but these effects are not associated with maternal weight loss. Behav. Brain Res. 213, 323–327 (2010).
Ozawa, K. et al. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol. Psychiatry 59, 546–554 (2006).
Buehler, M. R. A proposed mechanism for autism: an aberrant neuroimmune response manifested as a psychiatric disorder. Med. Hypotheses 76, 863–870 (2011).
Brown, A. S. & Derkits, E. J. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am. J. Psychiatry 167, 261–280 (2010).
Ashdown, H. et al. The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia. Mol. Psychiatry 11, 47–55 (2006).
Hsiao, E. Y. & Patterson, P. H. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav. Immun. 25, 604–615 (2011).
Brown, A. S. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev. Neurobiol. 72, 1272–1276 (2012).
Careaga, M., Murai, T. & Bauman, M. D. Maternal immune activation and autism spectrum disorder: from rodents to nonhuman and human primates. Biol. Psychiatry 81, 391–401 (2017).
Knox, K. & Baker, J. C. Genomic evolution of the placenta using co-option and duplication and divergence. Genome Res. 18, 695–705 (2008).
Winn, V. D. et al. Gene expression profiling of the human maternal-fetal interface reveals dramatic changes between midgestation and term. Endocrinology 148, 1059–1079 (2007).
Tanaka, T. S. et al. Genome-wide expression profiling of mid-gestation placenta and embryo using a 15,000 mouse developmental cDNA microarray. Proc. Natl. Acad. Sci. USA 97, 9127–9132 (2000).
Cox, B. et al. Comparative systems biology of human and mouse as a tool to guide the modeling of human placental pathology. Mol. Syst. Biol. 5, 279 (2009).
Nelissen, E. C. et al. Altered gene expression in human placentas after IVF/ICSI. Hum. Reprod. 29, 2821–2831 (2014).
Ursini, G. et al. Convergence of placenta biology and genetic risk for schizophrenia. Nat. Med. 24, 792–801 (2018).
Mouillet, J. F. et al. MicroRNAs in placental health and disease. Am. J. Obstet. Gynecol. 213, S163–S172 (2015).
Bianco-Miotto, T. et al. IFPA meeting 2015 workshop report I: placental mitochondrial function, transport systems and epigenetics. Placenta 48(Suppl. 1), S3–S6 (2016).
Jansson, T. & Powell, T. L. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin. Sci. (Lond.) 113, 1–13 (2007).
Bianco-Miotto, T. et al. Recent progress towards understanding the role of DNA methylation in human placental development. Reproduction 152, R23–R30 (2016).
McMullen, S. et al. Alterations in placental 11 beta-hydroxysteroid dehydrogenase (11 betaHSD) activities and fetal cortisol:cortisone ratios induced by nutritional restriction prior to conception and at defined stages of gestation in ewes. Reproduction 127, 717–725 (2004).
Murphy, V. E. & Clifton, V. L. Alterations in human placental 11beta-hydroxysteroid dehydrogenase type 1 and 2 with gestational age and labour. Placenta 24, 739–744 (2003).
Stirrat, L. I. et al. Transfer and metabolism of cortisol by the isolated perfused human placenta. J. Clin. Endocrinol. Metab. 103, 640–648 (2018).
Gomez-Roig, M. D. et al. Placental 11B-hydroxysteroid dehydrogenase type 2 mRNA levels in intrauterine growth restriction versus small-for-gestational-age fetuses. Fetal Diagn. Ther. 39, 147–151 (2016).
Brosens, I. et al. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am. J. Obstet. Gynecol. 204, 193–201 (2011).
Adelman, D. M. et al. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 14, 3191–3203 (2000).
Gheorghe, C. P. et al. Gene expression in the placenta: maternal stress and epigenetic responses. Int. J. Dev. Biol. 54, 507–523 (2010).
Januar, V. et al. Epigenetic regulation of human placental function and pregnancy outcome: considerations for causal inference. Am. J. Obstet. Gynecol. 213, S182–S196 (2015).
Mina, T. H. et al. Maternal distress associates with placental genes regulating fetal glucocorticoid exposure and IGF2: role of obesity and sex. Psychoneuroendocrinology 59, 112–122 (2015).
Davis, E. P., Waffarn, F. & Sandman, C. A. Prenatal treatment with glucocorticoids sensitizes the HPA axis response to stress among full-term infants. Dev. Psychobiol. 53, 175–183 (2011).
Straughen, J. K. et al. The association between placental histopathology and autism spectrum disorder. Placenta 57, 183–188 (2017).
Chang, J. M. et al. Autism risk classification using placental chorionic surface vascular network features. BMC Med. Inform. Decis. Mak. 17, 162 (2017).
Park B. Y. et al., National Children’s Study Council. Placental gross shape differences in a high autism risk cohort and the general population. PLoS ONE 13, e0191276 (2018).
Schmidt, R. J. et al. Self-reported pregnancy exposures and placental DNA methylation in the MARBLES prospective autism sibling study. Environ. Epigenet. 2, pii: dvw024 (2016).
Schroeder, D. I. et al. Placental methylome analysis from a prospective autism study. Mol. Autism 7, 51 (2016).
Bilbo, S. D. et al. Beyond infection—maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp. Neurol. 299, 241–251 (2018).
Wong, H. & Hoeffer, C. Maternal IL-17A in autism. Exp. Neurol. 299, 228–240 (2018).
Wu, W. L. et al. The placental interleukin-6 signaling controls fetal brain development and behavior. Brain Behav. Immun. 62, 11–23 (2017).
Ponzio, N. M. et al. Cytokine levels during pregnancy influence immunological profiles and neurobehavioral patterns of the offspring. Ann. NY Acad. Sci. 1107, 118–128 (2007).
Ali, A. et al. The placental immune response is dysregulated developmentally vitamin D deficient rats: relevance to autism. J. Steroid Biochem. Mol. Biol. 180, 73–80 (2018).
Vuillermot, S. et al. Vitamin D treatment during pregnancy prevents autism-related phenotypes in a mouse model of maternal immune activation. Mol. Autism 8, 9 (2017).
Anderson, P. & Doyle, L. W., Victorian Infant Collaborative Study Group. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA 289, 3264–3272 (2003).
Whitehouse, A. J. et al. Maternal vitamin D levels and the autism phenotype among offspring. J. Autism Dev. Disord. 43, 1495–1504 (2013).
Vinkhuyzen, A. A. E. et al. Gestational vitamin D deficiency and autism-related traits: the Generation R Study. Mol. Psychiatry 23, 240–246 (2018).
Vinkhuyzen, A. A. E. et al. Gestational vitamin D deficiency and autism spectrum disorder. BJPsych Open 3, 85–90 (2017).
Magnusson, C. et al. Maternal vitamin D deficiency and the risk of autism spectrum disorders: population-based study. BJPsych Open 2, 170–172 (2016).
Stubbs, G., Henley, K. & Green, J. Autism: Will vitamin D supplementation during pregnancy and early childhood reduce the recurrence rate of autism in newborn siblings? Med Hypotheses 88, 74–78 (2016).
Chawla, D. et al. Early prenatal vitamin D concentrations and social-emotional development in infants. J. Matern. Fetal Neonatal Med. 32, 1441–1448 (2019).
Garcia-Serna, A. M. & Morales, E. Neurodevelopmental effects of prenatal vitamin D in humans: systematic review and meta-analysis. Mol. Psychiatry (2019). https://doi.org/10.1038/s41380-019-0357-9.
Werling, D. M. & Geschwind, D. H. Sex differences in autism spectrum disorders. Curr. Opin. Neurol. 26, 146–153 (2013).
Robinson, E. B. et al. Examining and interpreting the female protective effect against autistic behavior. Proc. Natl. Acad. Sci. USA 110, 5258–5262 (2013).
Mueller, B. R. & Bale, T. L. Sex-specific programming of offspring emotionality after stress early in pregnancy. J. Neurosci. 28, 9055–9065 (2008).
Pankevich, D. E. et al. Prenatal stress programming of offspring feeding behavior and energy balance begins early in pregnancy. Physiol. Behav. 98, 94–102 (2009).
Bronson, S. L. & Bale, T. L. The placenta as a mediator of stress effects on neurodevelopmental reprogramming. Neuropsychopharmacology 41, 207–218 (2016).
Bale, T. L. The placenta and neurodevelopment: sex differences in prenatal vulnerability. Dialog. Clin. Neurosci. 18, 459–464 (2016).
Bronson, S. L., Chan, J. C. & Bale, T. L. Sex-specific neurodevelopmental programming by placental insulin receptors on stress reactivity and sensorimotor gating. Biol. Psychiatry 82, 127–138 (2017).
Ratto, A. B. et al. What about the girls? Sex-based differences in autistic traits and adaptive skills. J. Autism Dev. Disord. 48, 1698–1711 (2018).
Tang, S. et al. Altered forebrain functional connectivity and neurotransmission in a kinase-inactive met mouse model of autism. Mol. Imaging 18, 1536012118821034 (2019).
Sandman, C. A., Glynn, L. M. & Davis, E. P. Is there a viability-vulnerability tradeoff? Sex differences in fetal programming. J. Psychosom. Res. 75, 327–335 (2013).
Cumberland, A. L. et al. Increased anxiety-like phenotype in female guinea pigs following reduced neurosteroid exposure in utero. Int. J. Dev. Neurosci. 58, 50–58 (2017).
Fatemi, S. H. & Folsom, T. D. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr. Bull. 35, 528–548 (2009).
Fatemi, S. H. et al. The viral theory of schizophrenia revisited: abnormal placental gene expression and structural changes with lack of evidence for H1N1 viral presence in placentae of infected mice or brains of exposed offspring. Neuropharmacology 62, 1290–1298 (2012).
Fineberg, A. M. & Ellman, L. M. Inflammatory cytokines and neurological and neurocognitive alterations in the course of schizophrenia. Biol. Psychiatry 73, 951–966 (2013).
Shen, Q. et al. The role of pro-inflammatory factors in mediating the effects on the fetus of prenatal undernutrition: implications for schizophrenia. Schizophr. Res. 99, 48–55 (2008).
Cannon, M., Jones, P. B. & Murray, R. M. Obstetric complications and schizophrenia: historical and meta-analytic review. Am. J. Psychiatry 159, 1080–1092 (2002).
Plitman, E. et al. Kynurenic acid in schizophrenia: a systematic review and meta-analysis. Schizophr. Bull. 43, 764–777 (2017).
Beggiato, S. et al. Maternal genotype determines kynurenic acid levels in the fetal brain: implications for the pathophysiology of schizophrenia. J. Psychopharmacol. 32, 1223–1232 (2018).
Stroud, L. R. et al. Prenatal major depressive disorder, placenta glucocorticoid and serotonergic signaling, and infant cortisol response. Psychosom. Med. 78, 979–990 (2016).
Capron, L. E., Ramchandani, P. G. & Glover, V. Maternal prenatal stress and placental gene expression of NR3C1 and HSD11B2: the effects of maternal ethnicity. Psychoneuroendocrinology 87, 166–172 (2018).
Monk, C. et al. Distress during pregnancy: epigenetic regulation of placenta glucocorticoid-related genes and fetal neurobehavior. Am. J. Psychiatry 173, 705–713 (2016).
Raikkonen, K. et al. Maternal depressive symptoms during pregnancy, placental expression of genes regulating glucocorticoid and serotonin function and infant regulatory behaviors. Psychol. Med. 45, 3217–3226 (2015).
Cottrell, E. C. et al. Foetal and placental 11beta-HSD2: a hub for developmental programming. Acta Physiol. (Oxf.) 210, 288–295 (2014).
Wyrwoll, C. S. & Holmes, M. C. Prenatal excess glucocorticoid exposure and adult affective disorders: a role for serotonergic and catecholamine pathways. Neuroendocrinology 95, 47–55 (2012).
Bonnin, A. et al. A transient placental source of serotonin for the fetal forebrain. Nature 472, 347–350 (2011).
Janssen, A. B. et al. Maternal prenatal depression is associated with decreased placental expression of the imprinted gene PEG3. Psychol. Med. 46, 2999–3011 (2016).
Aarnoudse-Moens, C. S. et al. Executive function in very preterm children at early school age. J. Abnorm. Child Psychol. 37, 981–993 (2009).
Baron, I. S. et al. Cognitive deficit in preschoolers born late-preterm. Early Hum. Dev. 87, 115–119 (2011).
Talge, N. M. et al. Late-preterm birth by delivery circumstance and its association with parent-reported attention problems in childhood. J. Dev. Behav. Pediatr. 33, 405–415 (2012).
Penn, A. A. et al. Controversies in preterm brain injury. Neurobiol. Dis. 92, 90–101 (2016).
Guttmacher, A. E., Maddox, Y. T. & Spong, C. Y. The Human Placenta Project: placental structure, development, and function in real time. Placenta 35, 303–304 (2014).
Monk, D. Genomic imprinting in the human placenta. Am. J. Obstet. Gynecol. 213, S152–S162 (2015).
Simoes L. R., et al. Maternal immune activation induced by lipopolysaccharide triggers immune response in pregnant mother and fetus, and induces behavioral impairment in adult rats. J. Psychiatr Res. 100, 71–83 (2018).
Howerton, C. L., Morgan, C. P., Fischer, D. B., Bale, T. L. O-GlcNAc transferase (OGT) as a placental biomarker of maternal stress and reprogramming of CNS gene transcription in development. Proc. Natl Acad. Sci. USA 110, 5169–5174 (2013).
Guidotti, A., Grayson, D. R., Caruncho, H. J. Epigenetic RELN Dysfunction in Schizophrenia and Related Neuropsychiatric Disorders. Front Cell Neurosci. 10, 89 (2016).
Acknowledgements
This work was supported by NIH R01 (R01HD092593) and Simons Foundation Autism Research Initiative (SFARI) grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kratimenos, P., Penn, A.A. Placental programming of neuropsychiatric disease. Pediatr Res 86, 157–164 (2019). https://doi.org/10.1038/s41390-019-0405-9
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-019-0405-9
This article is cited by
-
Prenatal maternal stress in rats alters the epigenetic and transcriptomic landscape of the maternal-fetal interface across four generations
Communications Biology (2025)
-
Maternal stressors disrupt mouse placental proteome and fetal brain development in a sex-specific fashion through inflammation and oxidative stress
Molecular Psychiatry (2025)
-
Effects of preeclampsia on the hippocampus of offspring in rats
Neuroscience and Behavioral Physiology (2025)
-
Timing of antenatal corticosteroid exposure and its association with childhood mental disorders in early- and full-term births: A population-based cohort study
European Journal of Pediatrics (2025)
-
Impact of maternal immune activation and sex on placental and fetal brain cytokine and gene expression profiles in a preclinical model of neurodevelopmental disorders
Journal of Neuroinflammation (2024)