Abstract
Background
Prematurely born infants are frequently exposed to painful procedures in the neonatal intensive care unit, causing changes to the development of the nervous system lasting into adulthood. The current study aims to study acute and long-term consequences of neonatal repetitive noxious stimulation.
Methods
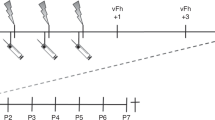
Rat pups received either 4 or 10 unilateral needle pricks per day, while control littermates received 4 or 10 tactile stimuli in the first postnatal week. Behavioural sensitivity was assessed in the neonatal phase, in adulthood, and after re-injury of the same dermatome in adulthood.
Results
An increase in the number of repetitive painful stimuli, from 4 to 10 needle pricks per day, resulted in increased mechanical hypersensitivity during the neonatal period. In adulthood, repetitive painful stimuli resulted in hyposensitivity to mechanical stimuli, while thermal sensitivity was unaffected. After re-injury of the same dermatome in adulthood, the number of repetitive noxious stimuli did not affect mechanical hypersensitivity. Both needle prick groups showed an increased duration of postoperative hypersensitivity compared to control.
Conclusion
This study shows that repetitive noxious stimulation during the early postnatal period affects acute and long-term mechanical sensitivity. Therefore, the amount of nociceptive stimuli should be minimized or adequately treated in a clinical setting.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Simons, S. H. et al. Do we still hurt newborn babies? A prospective study of procedural pain and analgesia in neonates. Arch. Pediatr. Adolesc. Med. 157, 1058–1064 (2003).
Carbajal, R. et al. Sedation and analgesia practices in neonatal intensive care units (EUROPAIN): results from a prospective cohort study. Lancet Respir. Med. 3, 796–812 (2015).
Roofthooft, D. W., Simons, S. H., Anand, K. J., Tibboel, D. & van Dijk, M. Eight years later, are we still hurting newborn infants? Neonatology 105, 218–226 (2014).
Carbajal, R. et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA 300, 60–70 (2008).
Walker, S. M. Neonatal pain. Paediatr. Anaesth. 24, 39–48 (2014).
Walker, S. M. Translational studies identify long-term impact of prior neonatal pain experience. Pain 158(Suppl 1), S29–S42 (2017).
Walker, S. M., Fitzgerald, M. & Hathway, G. J. Surgical injury in the neonatal rat alters the adult pattern of descending modulation from the rostroventral medulla. Anesthesiology 122, 1391–1400 (2015).
Fitzgerald, M. & Walker, S. M. Infant pain management: a developmental neurobiological approach. Nat. Clin. Pract. Neurol. 5, 35–50 (2009).
Brummelte, S. et al. Procedural pain and brain development in premature newborns. Ann. Neurol. 71, 385–396 (2012).
Grunau, R. E. et al. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain 143, 138–146 (2009).
de Graaf, J. et al. Five-year follow-up of effects of neonatal intensive care and morphine infusion during mechanical ventilation on diurnal cortisol rhythm. J. Pediatr. 165, 459–63 e2 (2014).
Beggs, S., Torsney, C., Drew, L. J. & Fitzgerald, M. The postnatal reorganization of primary afferent input and dorsal horn cell receptive fields in the rat spinal cord is an activity-dependent process. Eur. J. Neurosci. 16, 1249–1258 (2002).
Fitzgerald, M. & Jennings, E. The postnatal development of spinal sensory processing. Proc. Natl. Acad. Sci. USA 96, 7719–7722 (1999).
Duerden, E. G. et al. Early procedural pain is associated with regionally-specific alterations in thalamic development in preterm neonates. J. Neurosci. 38, 878–886 (2018).
Ranger, M. & Grunau, R. E. Early repetitive pain in preterm infants in relation to the developing brain. Pain Manag. 4, 57–67 (2014).
Anand, K. J., Coskun, V., Thrivikraman, K. V., Nemeroff, C. B. & Plotsky, P. M. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol. Behav. 66, 627–637 (1999).
Knaepen, L. et al. Neonatal repetitive needle pricking: plasticity of the spinal nociceptive circuit and extended postoperative pain in later life. Dev. Neurobiol. 73, 85–97 (2013).
Ruda, M. A., Ling, Q. D., Hohmann, A. G., Peng, Y. B. & Tachibana, T. Altered nociceptive neuronal circuits after neonatal peripheral inflammation. Science 289, 628–631 (2000).
Ren, K. et al. Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult. Pain 110, 588–596 (2004).
Jennings, E. & Fitzgerald, M. Postnatal changes in responses of rat dorsal horn cells to afferent stimulation: a fibre-induced sensitization. J. Physiol. 509(Pt 3), 859–868 (1998).
Laprairie, J. L. & Murphy, A. Z. Neonatal injury alters adult pain sensitivity by increasing opioid tone in the periaqueductal gray. Front. Behav. Neurosci. 3, 31 (2009).
Chen, M. et al. Neonatal repetitive pain in rats leads to impaired spatial learning and dysregulated hypothalamic-pituitary-adrenal axis function in later life. Sci. Rep. 6, 39159 (2016).
van den Hoogen, N. J. et al. Neonatal paracetamol treatment reduces long-term nociceptive behaviour after neonatal procedural pain in rats. Eur. J. Pain 20, 1309–1318 (2016).
van den Hoogen, N. J. et al. Repeated touch and needle-prick stimulation in the neonatal period increases the baseline mechanical sensitivity and postinjury hypersensitivity of adult spinal sensory neurons. Pain 159, 1166–1175 (2018).
Chaplan, S. R., Bach, F. W., Pogrel, J. W., Chung, J. M. & Yaksh, T. L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63 (1994).
Brennan, T. J., Vandermeulen, E. P. & Gebhart, G. F. Characterization of a rat model of incisional pain. Pain 64, 493–501 (1996).
Sternberg, W. F., Scorr, L., Smith, L. D., Ridgway, C. G. & Stout, M. Long-term effects of neonatal surgery on adulthood pain behavior. Pain 113, 347–353 (2005).
Ren, K., Novikova, S. I., He, F., Dubner, R. & Lidow, M. S. Neonatal local noxious insult affects gene expression in the spinal dorsal horn of adult rats. Mol. Pain 1, 27 (2005).
van den Hoogen, N. J., van Reij, R. R., Patijn, J., Tibboel, D. & Joosten, E. A. J. Adult spinal opioid receptor mu1 expression after incision is altered by early life repetitive tactile and noxious procedures in rats. Dev. Neurobiol. 78, 417–426 (2018).
Butkevich, I. P., Mikhailenko, V. A., Vershinina, E. A. & Aloisi, A. M. Effects of neonatal pain, stress and their interrelation on pain sensitivity in later life in male rats. Chin. J. Physiol. 59, 225–231 (2016).
Weaver, S. A., Diorio, J. & Meaney, M. J. Maternal separation leads to persistent reductions in pain sensitivity in female rats. J. Pain 8, 962–969 (2007).
Schwaller, F. & Fitzgerald, M. The consequences of pain in early life: injury-induced plasticity in developing pain pathways. Eur. J. Neurosci. 39, 344–352 (2014).
Peters, J. W. et al. Does neonatal surgery lead to increased pain sensitivity in later childhood? Pain 114, 444–454 (2005).
Simons, S. H. & Tibboel, D. Pain perception development and maturation. Semin. Fetal Neonatal Med. 11, 227–231 (2006).
Van den Hoogen, N. J., Patijn, J., Tibboel, D. & Joosten, E. A. Neonatal plasticity of the nociceptive system: mechanisms, effects, and treatment of repetitive painful procedures during NICU admittance. Curr. Pharm. Des. 23, 5902–5910 (2017).
Acknowledgements
This research was financially supported by the Pain Knowledge Centers from Maastricht and Rotterdam.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
van den Hoogen, N.J., Patijn, J., Tibboel, D. et al. Repetitive noxious stimuli during early development affect acute and long-term mechanical sensitivity in rats. Pediatr Res 87, 26–31 (2020). https://doi.org/10.1038/s41390-019-0420-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41390-019-0420-x
This article is cited by
-
Repetitive neonatal pain increases spinal cord DNA methylation of the µ-opioid receptor
Pediatric Research (2025)
-
Repetitive daily oxytocin treatment reduces weight gain but not acute neonatal procedural pain
Pediatric Research (2025)
-
The development of descending serotonergic modulation of the spinal nociceptive network: a life span perspective
Pediatric Research (2022)
-
Methadone effectively attenuates acute and long-term consequences of neonatal repetitive procedural pain in a rat model
Pediatric Research (2021)
-
Insights Image for “Repetitive noxious stimuli during early development affect acute and long-term mechanical sensitivity in rats”
Pediatric Research (2020)