Abstract
Background
We evaluated the association between etiology of maternal anemia and iron status throughout infancy.
Methods
Samples from a study designed to examine Praziquantel treatment during pregnancy were used (n = 359). All women were infected with schistosomiasis and randomized to Praziquantel or placebo at 16 ± 2 weeks’ gestation. Hemoglobin, serum ferritin (SF), soluble transferrin receptor (sTfR), hepcidin, C-reactive protein, and interleukin-6 were measured in maternal and infant blood. The relationship between both maternal Praziquantel treatment and etiology of anemia and infant iron status was evaluated.
Results
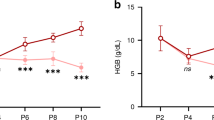
Maternal iron-deficiency anemia was associated with increased risk of infant anemia at 6 months of age. Infants of mothers with the lowest levels of circulating hepcidin during gestation, likely a marker for iron deficiency, had higher sTfR:SF levels and lower hemoglobin levels, particularly at 12 months of age. Maternal non-iron-deficiency anemia (NIDA) did not impact infant anemia risk or iron status. Maternal treatment for schistosomiasis had no effect on infant hematologic status.
Conclusions
Maternal iron deficiency anemia was associated with an increased risk for anemia or iron deficiency during late infancy. We did not observe an association between maternal NIDA and increased risk for iron deficiency during infancy.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Stevens, G. et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnancy and non-pregnant women for 1995-2011: a systematic analysis of population-representative data. Lancet Glob. Health 1, e16–e25 (2013).
Menon, K. et al. Effects of anemia at different stages of gestation on infant outcomes. Nutrition 32, 61–65 (2016).
Clardy, S. L. et al. Acute and chronic effects of developmental iron deficiency on mRNA expression patterns in the brain. J. Neural Transm. Suppl. 173–196 (2006).
de Deungria, M. et al. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatr. Res. 48, 169–176 (2000).
Felt, B. T. & Lozoff, B. Brain iron and behavior of rats are not normalized by treatment of iron deficiency anemia during early development. J. Nutr. 126, 693–701 (1996).
Georgieff, M. K. Long-term brain and behavioral consequences of early iron deficiency. Nutr. Rev. 69(Suppl 1), S43–S48 (2011).
Petry, N. et al. The proportion of anemia associated with iron deficiency in low, medium, and high human development index countries: a systematic analysis of national surveys. Nutrients 8, E693 (2016).
Park, S. et al. Mechanistic pathways from early gestation through infancy and neurodevelopment. Pediatrics 138, e20161843 (2016).
El-Farrash, R. A., Ismail, E. A. & Nada, A. S. Cord blood iron profile and breast milk micronutrients in maternal iron deficiency anemia. Pediatr. Blood Cancer 58, 233–238 (2012).
Kumar, A., Rai, A. K., Basu, S., Dash, D. & Singh, J. S. Cord blood and breast milk iron status in maternal anemia. Pediatrics 121, e673–e677 (2008).
Hay, G. et al. Predictors of serum ferritin and serum soluble transferrin receptor in newborns and their associations with iron status during the first 2 y of life. Am. J. Clin. Nutr. 86, 64–73 (2007).
Abioye, A. I. et al. Anemia of inflammation during human pregnancy does not affect newborn iron endowment. J. Nutr. 148, 427–436 (2018).
Ziegler, E. E., Nelson, S. E. & Jeter, J. M. Iron stores of breastfed infants during the first year of life. Nutrients 6, 2023–2034 (2014).
Wang, C. Y. & Babitt, J. L. Hepcidin regulation in the anemia of inflammation. Curr. Opin. Hematol. 23, 189–197 (2016).
Nicolas, G. et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc. Natl Acad. Sci. USA 99, 4596–4601 (2002).
Ganz, T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 102, 783–788 (2003).
Koenig, M. D., Tussing-Humphreys, L., Day, J., Cadwell, B. & Nemeth, E. Hepcidin and iron homeostasis during pregnancy. Nutrients 6, 3062–3083 (2014).
Leenstra, T. et al. Schistosomiasis japonica, anemia, and iron status in children, adolescents, and young adults in Leyte, Philippines 1. Am. J. Clin. Nutr. 83, 371–379 (2006).
Leenstra, T. et al. Schistosoma japonicum reinfection after praziquantel treatment causes anemia associated with inflammation. Infect. Immun. 74, 6398–6407 (2006).
Friedman, J. F., Kanzaria, H. & McGarvey, S. T. Human schistosomiasis and anemia: the relationship and potential mechanisms. Trends Parasitol. 21, 386–392 (2005).
Onguru, D. et al. Human schistosomiasis is associated with endotoxemia and Toll-like receptor 2- and 4-bearing B cells. Am. J. Trop. Med. Hyg. 84, 321–324 (2011).
Olveda, R. et al. Efficacy and safety of praziquantel for the treatment of human schistosomiasis during pregnancy: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 16, 199–208 (2016).
WHO. Prevention and control of intestinal parasitic infections. World Health Organ Tech. Rep. Ser. 749, 1–86 (1987).
Coutinho, H. et al. Nutritional status and serum cytokine profiles in children, adolescents, and young adults with Schistosoma japonicum-associated hepatic fibrosis, in Leyte, Philippines. J. Infect. Dis. 192, 528–536 (2005).
FDA. Quantikine sTfR IVD Kit: 510(k) Substantial Equivalence Determination Decision Summary (US Food and Drug Administration, Silver Spring, MD, 2008).
WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System (World Health Organization, Geneva, 2011).
WHO. Serum Ferritin Concentrations for the Assessment of Iron Status and Iron Deficiency in Populations. Vitamin and Mineral Nutrition Information System (World Health Organization, Geneva, 2011).
van den Broek, N., Letsky, E., White, S. & Shenkin, A. Iron status in pregnant women: which measurements are valid? Br. J. Haematol. 103, 817–824 (1998).
Shin, D., Kim, H., Park, M., Suh, I. & Shin, K. Utility of access soluble transferrin receptor (sTfR) and sTfR/log Ferritin Index in diagnosing iron deficiency anemia. Ann. Clin. Lab. Sci. 45, 396–402 (2015).
Phiri, K. S. et al. New cut-off values for ferritin and soluble transferrin receptor for the assessment of iron deficiency in children in a high infection pressure area. J. Clin. Pathol. 62, 1103–1106 (2009).
Coutinho, H. et al. Pro-inflammatory cytokines and C-reactive protein are associated with undernutrition in the context of Schistosoma japonicum infection. Am. J. Trop. Med. Hyg. 75, 720–726 (2006).
Coutinho, H. M. et al. Nutritional status improves after treatment of schistosoma japonicum-infected children and adolescents. J. Nutr. 136, 183–188 (2006).
Raiten, D. et al. Executive summary--biomarkers of nutrition for development: building a consensus. Am. J. Clin. Nutr. 94, 633S–650SS (2011).
Scholl, T. O. Maternal iron status: relation to fetal growth, length of gestation, and iron endowment of the neonate. Nutr. Rev. 69, S23–S29 (2011).
Abioye, A. I. et al. Iron supplementation affects hematologic biomarker concentrations and pregnancy outcomes among iron-deficient Tanzanian women. J. Nutr. 146, 1162–1171 (2016).
Gambling, L. et al. Fetal iron status regulates maternal iron metabolism during pregnancy in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R1063–R1070 (2009).
Lubach, G. & Coe, C. Preconception maternal iron status is a risk factor for iron deficiency in infant rhesus monkeys (Macaca mulatta). J. Nutr. 136, 2345–2349 (2006).
Lozoff, B. et al. Low-dose iron supplementation in infancy modestly increases infant iron status at 9 mo without decreasing growth or increasing illness in a randomized clinical trial in rural China. J. Nutr. 146, 612–621 (2016).
Uyoga, M. et al. Duration of exclusive breastfeeding is a positive predictor of iron status in 6- to 10-month-old infants in rural Kenya. Matern. Child Nutr. 13, https://doi.org/10.1111/mcn.12386 (2017).
Acknowledgements
We thank our study participants, dedicated field staffs in Leyte, The Philippines and our funders (NIH/NIAID and Thrasher Research Fund) for supporting this work. The randomized controlled trial was supported by NIH/NIAID, “S. japonicum and pregnancy outcomes: An RCT” (U01AI066050) with relevant data for this manuscript collected through newborn day of life 28. NIH/NIAID (R21AI107520) “S. japonicum, anemia, and iron transport in human pregnancy” supported extended biomarkers to define etiology of anemia among pregnant women and their offspring.
Author information
Authors and Affiliations
Contributions
All the authors participated in the planning and design of the study. M.J.S., A.J.A., P.I.B., V.T., L.P.A. and R.M.O. participated in data collection. E.A.M., H.W.W., S.P.-T., and J.D.K. did the laboratory assays. A.I.A. and S.P. analyzed data. A.I.A., E.A.M. and J.F.F. participated in the primary manuscript writing. J.F.F. had primary responsibility for the final content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abioye, A.I., McDonald, E.A., Park, S. et al. Maternal anemia type during pregnancy is associated with anemia risk among offspring during infancy. Pediatr Res 86, 396–402 (2019). https://doi.org/10.1038/s41390-019-0433-5
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41390-019-0433-5
This article is cited by
-
The effect of iron supplementation on maternal iron deficiency anemia does not differ by baseline anemia type among Tanzanian pregnant women without severe iron deficiency anemia
European Journal of Nutrition (2022)
-
Maternal health status and household food security on determining childhood anemia in Bangladesh -a nationwide cross-sectional study
BMC Public Health (2021)